Figures & data
Figure 1. Disease progression in Hgsnat-Geo mice. (A) Six and 8-month-old Hgsnat-Geo female mice show signs of hyperactivity and reduced anxiety compared to WT mice as detected by open field test performed 1 h into their light cycle. Increased activity (total distance traveled) was detected at 8 months. Reduced anxiety (increased center activity) was detected at 6 and 8 months. P value was calculated by 2-way-ANOVA (*P < 0.05, **P < 0.01). From 6 (2, 4 and 6 month old) to 10 (8 and 10 month old) naive mice were studied per age/per genotype. (B) Hgsnat-Geo mice (N = 6; 3 males, 3 females) showed impaired performance in the spatial memory-based Morris Water Maze test at 10 months. All mice showed similar average latencies on days 1–3 of visible platform testing. Whereas 8-month-old Hgsnat-Geo mice had latencies in the hidden platform testing similar to those of their wild type counterparts (days 4–8), 10-month-old mutant mice were significantly impaired in this spatial learning test. (C). Kaplan-Meier plot showing survival of Hgsnat-Geo mice (N = 50; 25 males, 25 females) and their wild type counterparts (N = 70; 35 males, 35 females). By the age of 70 weeks the vast majority of Hgsnat-Geo mice died or had to be euthanized on the veterinarian request due to urinary retention.
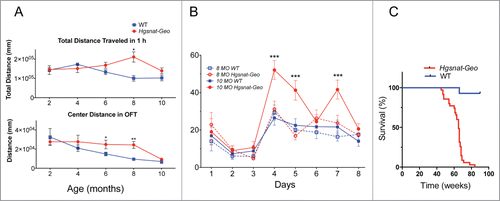
Figure 2. Pathological changes in the brains of Hgsnat-Geo mice. (A) Progressive loss of neurons in somatosensory cortex. NeuN-positive neurons were counted in 2 adjacent fields on 3 sagittal sections (1.44, 1.68 and 1.92 mm from bregma) of S1 somatosensory cortex; 2 male and 2 female mice were studied for each age and each genotype. Two-way repeated measurements ANOVA was used to test differences between the mouse groups: significant differences between the mean values in Bonferroni post-test (*P < 0.05, **P < 0.001, ***P < 0.0001) are shown. (B) Purkinje cells are largely absent in the lobule III of the anterior cerebellar lobe of Hgsnat-Geo mouse at the age of 12 months. H&E staining. Bar represents 50 µm. (C) Increased numbers of GFAP-positive astrocytes (red) and CD68-positive microglia (green) are detected in the somatosensory cortex of 4 months-old Hgsnat-Geo mice. (D) Storage pattern in microglia detected at 5 months. Massive accumulation of vacuoles with single limiting membranes and a sparse fine content in the cytoplasm is compatible with lysosomal GAG storage. Lysosomes containing storage materials are marked by arrowheads. The microglial cell (nucleus is marked by an asterisk) is in a close proximity to a cortical brain neuron. Bar represents 2 µm. (E) Lysosomal system in a cortical neuron at 12 months is expanded and massively overloaded by electron dense material (marked by arrows). Bar represents 2 µm. (F) Mitochondrial population in neurons of WT mice at the age of 12 months is relatively uniform and is composed of normally shaped mitochondria with largely regular cristae (details are shown in the insert). Bar represent 1 µm. (E) At the age of 12 months mitochondria in neurons of Hgsnat-Geo mice are displaying swelling and disorganization of their inner membranes (marked by arrowheads). Edematous mitochondria with largely dissolved cristae are marked by asterisks. Insert shows a detailed view of highly edematous mitochondria with remnants of double membranes (marked by double arrowheads). Bar represents 1 µm.
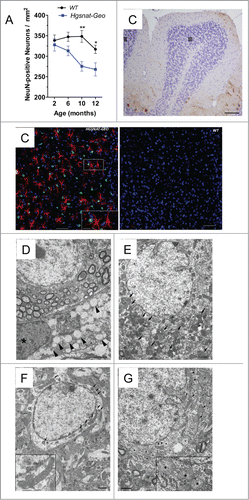
Figure 3. Proposed mechanism underlying brain disease in Hgsnat-Geo mice. The disease starts with accumulation of HS and HS-derived oligosaccharides in microglial cells (1). Through their release by exocytosis of lysosomes and action on TLR receptors (2) these materials induce general inflammation reactions in the brain, including the release of multifunctional cytokines such as TNFα and MIP-1α (3). The cytokines cause mitochondrial damage (4), and together with primary storage contribute to autophagy block and secondary storage of gangliosides and missfolded proteins in neurons (5) eventually leading to neuronal death in critical areas (6).