Figures & data
Figure 1. Proposed molecular defects in Rad3 ATP-binding groove mutants that may help understand XP/CS. The severity of the ATPase defect of rad3/XPD mutants would correlate with both its incapacity of DNA bubble opening and its ssDNA affinity. Mutants more affected were presumed to be the most UV-sensitive, rad3–2 and rad3–107, whereas rad3–101 and rad3–102 would display an intermediate defect, thus allowing eventual opening of the damaged DNA and a major permanence of the TFIIH complex at the DNA.
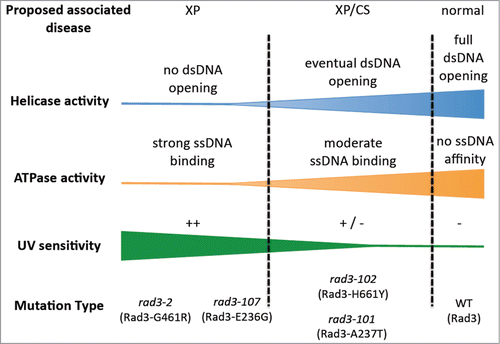
Figure 2. TFIIH dynamics in Rad3 ATP-binding groove mutants. Representation of FRAP curves normalized to a global maximum of Tfb4-yGFP strains after 80 J/m2 UV-C irradiation. Recovery curves of the normalized fluorescence in wild-type (WT) and rad3 mutants affected in the ATP-binding groove of Rad3 (rad3–102 [Rad3-H661Y], rad3–101 [Rad3-A237T], rad3–107 [Rad3-E236G] and rad3–2 [Rad3-G461R]) are displayed (adapted from data in ref.Citation9). The different steepness of the linear part of the curves shows the average molecular weight of the TFIIH complex under measurement.
![Figure 2. TFIIH dynamics in Rad3 ATP-binding groove mutants. Representation of FRAP curves normalized to a global maximum of Tfb4-yGFP strains after 80 J/m2 UV-C irradiation. Recovery curves of the normalized fluorescence in wild-type (WT) and rad3 mutants affected in the ATP-binding groove of Rad3 (rad3–102 [Rad3-H661Y], rad3–101 [Rad3-A237T], rad3–107 [Rad3-E236G] and rad3–2 [Rad3-G461R]) are displayed (adapted from data in ref.Citation9). The different steepness of the linear part of the curves shows the average molecular weight of the TFIIH complex under measurement.](/cms/asset/b5c1549b-f989-4eb2-8ea5-f126d36519f8/krad_a_1079362_f0002_oc.gif)
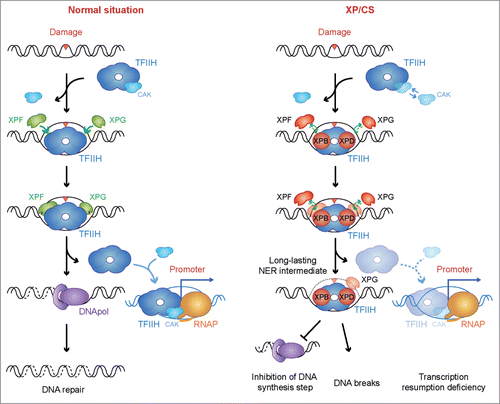
Figure 3. A possible unified model to explain the XP/CS molecular defect. In a normal situation, TFIIH is recruited to the damaged site, releasing the CAK subcomplex and allowing DNA unwinding and excision of the DNA containing the lesion by the XPF and XPG endonucleases. After DNA repair completion, TFIIH is relocated to the promoter sites, thus allowing transcription resumption. In XP/CS cells, TFIIH is also recruited to the damaged site and performs bubble opening. Incision by XPF or XPG nucleases could be prevented. In all cases, TFIIH could be retained in the DNA as a long-lasting NER intermediate. As a consequence, DNA synthesis would be impaired and DNA break formation may occur whenever incision has happened. TFIIH retention would provoke a delay in transcription resumption. In this scenario, an excess of free CAK is a possible outcome of some XP/CS mutations. Proteins whose deficiencies can cause an XP/CS condition are highlighted in red: XPB, XPD, XPF and XPG.