Figures & data
Figure 1. Comparison of 2 different strategies to study human disease using neurons derived from pluripotent stem cells. (A) The patient-approach uses iPS cells from a patient with a pathogenic mutation. iPS cells are differentiated into neurons and analyzed. The matching control neurons are derived from pluripotent stem cells after correction of the mutation by genetic engineering and clonal selection, or are derived from an unrelated healthy control person. (B) The conditional approach starts with pluripotent stem cells from a healthy person without mutations. The disease-relevant mutation is introduced into the stem cells as a conditional allele by genetic engineering and clonal selection, with an intermediate state in which the targeted gene contains a resistance cassette that is then removed by Flp-recombination as shown. The conditionally mutant stem cells are differentiated into neurons with simultaneous expression of mutant Cre-recombinase (as a control) or wild-type Cre-recombinase (to conditionally delete the floxed exon and produce a loss-of-function state). An alternative way is to use Flp-recombined cells as control neurons and Cre-recombined as mutant neurons.
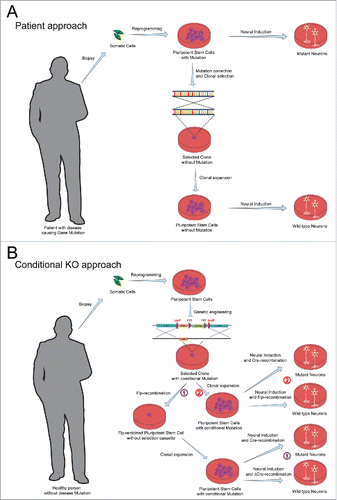
Figure 2. Gene-targeting of the STXBP1 gene encoding Munc18-1, and reduced synaptic transmission in heterozygous STXBP1 mutant neurons. (A) Using homologous recombination, Exon 2 (encompassing 50 bp of coding sequence 3′ of the translational start codon) is flanked by loxP sites and a resistance cassette for Puromycin or Blastocidin surrounded by frt sites is inserted into the intron 5′ of exon 2. Excision of the resistance cassette by flp recombinase restores the wild-type gene containing loxP sites 3′ and 5′ of exon 2. However, Cre-recombination excises exon 2, causing a frameshift that leads to a loss-of-function mutation of STXBP1. [Taken from Citation6, ]. (B) Paired-recordings from synaptically coupled neurons using optogenetics. Human neurons are sparsely transfected with tdTomato-tagged CHIEF (a channelrhodopsin-2 variant), and stimulated by blue light. Synaptic responses are measured from an untransfected adjacent post-synaptic neuron. (C)Decreased light-evoked synaptic transmission in heterozygous STXBP1 mutants. Co-transfection of wild-type rat Munc18-1 with channelrhodopsin into the heterozygous STXBP1-mutant neurons rescues the heterozygous phenotype. [B and C are taken from Citation6, Fig. 6E-F].
![Figure 2. Gene-targeting of the STXBP1 gene encoding Munc18-1, and reduced synaptic transmission in heterozygous STXBP1 mutant neurons. (A) Using homologous recombination, Exon 2 (encompassing 50 bp of coding sequence 3′ of the translational start codon) is flanked by loxP sites and a resistance cassette for Puromycin or Blastocidin surrounded by frt sites is inserted into the intron 5′ of exon 2. Excision of the resistance cassette by flp recombinase restores the wild-type gene containing loxP sites 3′ and 5′ of exon 2. However, Cre-recombination excises exon 2, causing a frameshift that leads to a loss-of-function mutation of STXBP1. [Taken from Citation6, Fig. 1A]. (B) Paired-recordings from synaptically coupled neurons using optogenetics. Human neurons are sparsely transfected with tdTomato-tagged CHIEF (a channelrhodopsin-2 variant), and stimulated by blue light. Synaptic responses are measured from an untransfected adjacent post-synaptic neuron. (C)Decreased light-evoked synaptic transmission in heterozygous STXBP1 mutants. Co-transfection of wild-type rat Munc18-1 with channelrhodopsin into the heterozygous STXBP1-mutant neurons rescues the heterozygous phenotype. [B and C are taken from Citation6, Fig. 6E-F].](/cms/asset/317e38cb-afdf-4276-84ee-114b68bb618d/krad_a_1131884_f0002_oc.gif)
