Figures & data
Figure 1. Trial profile. *4 discontinued treatment (2 patient’s request [Cycle 4], 1 adverse event [Cycle 5], 1 hard to visit the hospital due to worsening of ALS [Cycle 5]).
![Figure 1. Trial profile. *4 discontinued treatment (2 patient’s request [Cycle 4], 1 adverse event [Cycle 5], 1 hard to visit the hospital due to worsening of ALS [Cycle 5]).](/cms/asset/270fca6f-d776-4d70-b9cf-2b0aa6cf51bd/iafd_a_1361441_f0001_b.jpg)
Table 1. Subject demographic characteristics in the FAS.
Table 2. Change in endpoints during treatment in the FAS.
Figure 2. Mean ALSFRS-R scores through the study period. For patients with missing values at the end of Cycle 6, data were imputed by the LOCF method, provided that they had completed at least Cycle 3. ALS: amyotrophic lateral sclerosis. ALSFRS-R: Revised ALS Functional Rating Scale. LOCF: last observation carried forward; SD: standard deviation.
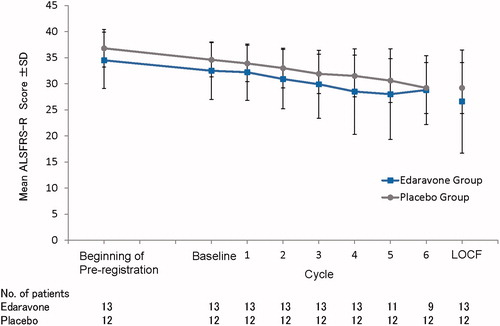
Figure 3. Changes of the ALSFRS-R score during treatment in individual patients. ALSFRS-R: revised amyotrophic lateral sclerosis functional rating scale. Change of the ALSFRS-R score defined as the change from baseline to the end of Cycle 6 (or at discontinuation). For patients with missing values at the end of Cycle 6, data were input by the last observation carried forward (LOCF) method, provided that they had completed at least Cycle 3. •: change in the ALSFRS-R score during pre-observation period: −1, −2. ▪: change in the ALSFRS-R score during pre-observation period: −3, −4.
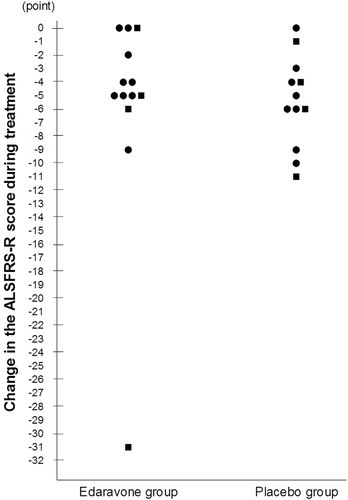
Table 3. Events of death or a specified state of disease progression in the FAS.
Table 4. Adverse events that occurred in more than two patients in either group (safety analysis set).
Table 5. All serious adverse events in the safety analysis set.
