Figures & data
Table 1. Disposition of patients in each population.
Table 2. Demographics and baseline characteristics of the dpEESP2y population.
Figure 1. Forest plots of between-group differences in least-squares mean (Δ LS-mean) of ALSFRS-R change in the FAS, EESP, and dpEESP2y during double-blind treatment.
1a. From baseline to week 24 of study MCI186-16; 1b from week 24 to week 48 of study MCI186-17.
FAS = full analysis set; EESP = efficacy-expected sub-population of ALS patients (% forced vital capacity of ≥80% before treatment and ≥2 points for all item scores in the ALSFRS-R before treatment); dpEESP2y = subgroup of the EESP, containing patients with a diagnosis of ‘definite’ or ‘probable’ ALS according to the El Escorial revised Airlie House diagnostic criteria and with disease duration of ≤2 years; ALSFRS-R = revised ALS functional rating scale; CI = confidence interval; SE = standard error.
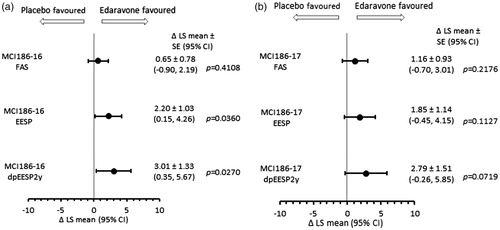
Table 3. Changes in ALSFRS-R scores in study MCI186-16 and subsequent study MCI186-17 in FAS, subpopulation of each criterion in FAS, EESP and dpEESP2y (LOCF).
Figure 2. Mean ALSFRS-R score ± standard deviation (SD) by treatment cycle in the dpEESP2y in studies MCI186-16 and MCI186-17.
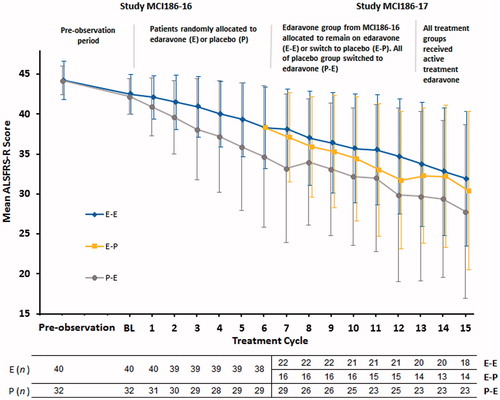
Table 4. Analysis of other efficacy endpoints in the dpEESP2y (Change from baseline of cycle 7 to the end of cycle 12) (LOCF).
Table 5. Adverse events reported in at least two patients in any treatment group: cycle 7 to cycle 15.
Table 6. Serious adverse events reported in any treatment group: cycle 7 to cycle 15.
