Figures & data
Figure 1. Study design.
This article reports this results of the extension study (Double blind period: Cycles 7–12, and Open label period: Cycles 13–15) indicated by the bold frame.
*As unblinding of the first phase III study was performed during this extension study, the blind was only maintained for the patients who were assigned to the E group in the first phase III study, but not for the patients who were assigned to the P group in the first phase III study.
Figure 2. Disposition of patients.
FAS: Full analysis set, EESP: Efficacy-expected sub-population of ALS patients in the FAS (forced vital capacity of at least 80% before treatment and at least 2 points for all item scores in ALSFRS-R before treatment).
ALS: amyotrophic lateral sclerosis, ALSFRS-R: revised ALS functional rating scale.
Abbreviations of groups are as follows: E-E, edaravone group (the first phase III study) - edaravone group (this extension study); E-P, edaravone group (the first phase III study) - placebo group (this extension study); P-E, placebo group (the first phase III study) - edaravone group (this extension study).
Detailed reasons for discontinuation (numbers are numbers of patients):
*: patient's withdrawal request.
**: patient's request, E-E 2, E-P 4, P-E 5; adverse event, E-E 3, E-P 2, P-E 2; tracheotomy, E-E 7, E-P 1, P-E 6; patient's convenience, E-E 0, E-P 0, P-E 1; other reasons, E-E 2, E-P 0, P-E 2.
***: diagnosed as not having ALS. ****: patient's request, E-P 2, P-E 2; adverse event, E-E 1, tracheotomy, E-E 2, P-E 2; P-E 1; other reasons, P-E 2.
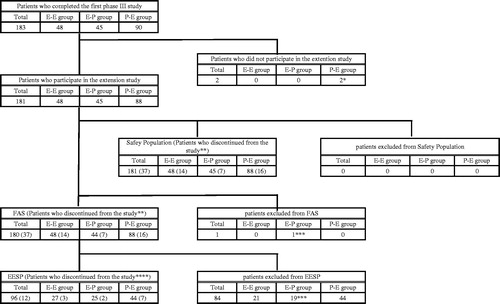
Table 1. Subject demographic characteristics in the FAS.
Table 2. Subject demographic characteristics in the EESP.
Figure 3. Changes in ALSFRS-R score in the first phase III study and this extension study in the FAS (a) and the EESP (b).
FAS: full analysis set
EESP: efficacy-expected sub-population in the FAS (forced vital capacity of at least 80% at baseline in Cycle 1 and at least 2 points for all item scores in ALSFRS-R).
ALS: amyotrophic lateral sclerosis.
ALSFRS-R: revised ALS functional rating scale.
In the first phase III study (from before pre-registration to the end of Cycle 6), the groups were as follows: E, edaravone group; P, placebo group.
In this extension study (baseline in Cycle 7 to the end of Cycle 15), the groups were as follows: E-E, edaravone group (the first phase III study) - edaravone group (this extension study); E-P, edaravone group (the first phase III study) - placebo group (this extension study); P-E, placebo group (the first phase III study) - edaravone group (this extension study).
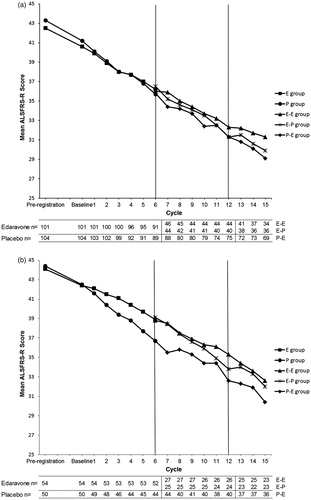
Table 3. Changes in endpoints from baseline in Cycle 7 to the end of Cycle 12 in the FAS and the EESP.
Table 4. Events of death or a specified state of disease progression in the FAS and the EESP.
Table 5. Adverse events in the Safety Analysis Set.
Table 6. All serious adverse events in the Safety Analysis Set.
