Figures & data
Figure 1. Trial profile. *Patient’s request, 2; **Patient’s request, 2; ***Patient’s request, 6; investigator’s decision due to AE, 1 (pneumonia aspiration); investigator’s decision due to worsening of ALS, 1; %FVC of ≤50% and PaCO2 (blood gas) of ≥45 mmHg, 4; ****patient’s request, 7; investigator’s decision due to AE, 2 (pneumonia aspiration, blood in urine/protein in urine/blood pressure increased); all-day respiratory support required, 3; %FVC of ≤50% and PaCO2 (blood gas) of ≥45 mmHg, 6. AE: adverse event; %FVC: % forced vital capacity.
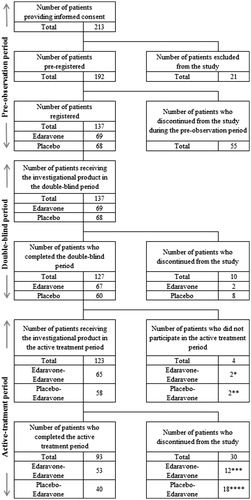
Table 1. Subject demographic characteristics in the FAS.
Figure 2. The efficacy endpoints in the FAS (Mean ± SD). (a) ALSFRS-R score (b) %FVC (c) Modified Norris Scale (d) ALSAQ-40; ALSFRS-R score: Total =48, Worst =0, Best =48; ALSFRS-R score (Limb function): Total =24, Worst =0, Best =24; ALSFRS-R score (Bulbar function): Total =12, Worst =0, Best =12; ALSFRS-R score (Respiratory function): Total =12, Worst =0, Best =12; Modified Norris Scale score: Total =102, Worst =0, Best =102; Modified Norris Scale score (Limb scale): Total =63, Worst =0, Best =63; Modified Norris Scale score (Bulbar scale): Total =39, Worst =0, Best =39; ALSAQ-40 score: Total =200, Worst =200, Best =40. BL: baseline; FAS: full analysis set; SD: standard deviation; ALSFRS-R: Revised ALS Functional Rating Scale; %FVC: % forced vital capacity; ALSAQ-40: ALS assessment questionnaire 40; E: Edaravone group; P: placebo group.
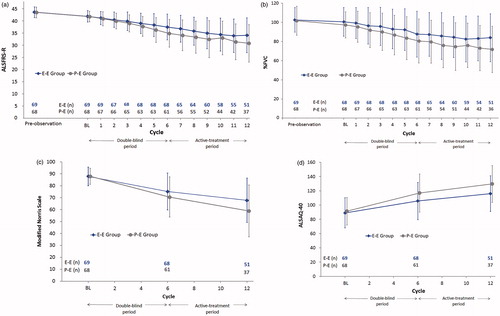
Figure 3. Survival rate, by death or a specified state of disease progression* in the FAS. A Kaplan-Meier curve was constructed, with any of ‘Death, Disability of independent ambulation, Loss of upper limbs function, Tracheotomy, Use of respirator, Use of tube feeding and Loss of useful speech’ defined as an event, and the date of censoring, which was defined as the last observation date. For patients with multiple events, the day of onset of the first event was considered the event onset day. *Event description. Death. Disability of independent ambulation: Rating of 0 points (‘No purposeful leg movement’) for ALSFRS-R item ‘8. Walking’ was used as criterion. Loss of upper limbs function: Criterion was ALSFRS-R rating of 0 points for all of the following items: ‘Handwriting,’ ‘Eating motion,’ and ‘Dressing and hygiene’ (i.e., ‘Handwriting’ [‘Unable to grip pen’]; ‘Eating motion: Handling utensils (patients without gastrostomy)’ [‘Needs to be fed’]; ‘Eating motion: Finger motion (patients with gastrostomy)’ [‘Unable to perform any aspect of task’]; and ‘Dressing and hygiene’ [‘Total dependence’]). Tracheotomy. Use of respirator: Did not include the use of BiPAP. Use of tube feeding: Criterion was ALSFRS-R rating of 0 points for ‘Swallowing’ (Exclusively parenteral or enteral feeding). Loss of useful speech: Criterion was ALSFRS-R rating of 0 points for ‘Speech’ (Loss of useful speech). Abbreviations: ALSFRS-R: ALS functional rating scale-revised; BiPAP: bilevel positive airway pressure; FAS: full analysis set; E: edaravone group; P: placebo group.
![Figure 3. Survival rate, by death or a specified state of disease progression* in the FAS. A Kaplan-Meier curve was constructed, with any of ‘Death, Disability of independent ambulation, Loss of upper limbs function, Tracheotomy, Use of respirator, Use of tube feeding and Loss of useful speech’ defined as an event, and the date of censoring, which was defined as the last observation date. For patients with multiple events, the day of onset of the first event was considered the event onset day. *Event description. Death. Disability of independent ambulation: Rating of 0 points (‘No purposeful leg movement’) for ALSFRS-R item ‘8. Walking’ was used as criterion. Loss of upper limbs function: Criterion was ALSFRS-R rating of 0 points for all of the following items: ‘Handwriting,’ ‘Eating motion,’ and ‘Dressing and hygiene’ (i.e., ‘Handwriting’ [‘Unable to grip pen’]; ‘Eating motion: Handling utensils (patients without gastrostomy)’ [‘Needs to be fed’]; ‘Eating motion: Finger motion (patients with gastrostomy)’ [‘Unable to perform any aspect of task’]; and ‘Dressing and hygiene’ [‘Total dependence’]). Tracheotomy. Use of respirator: Did not include the use of BiPAP. Use of tube feeding: Criterion was ALSFRS-R rating of 0 points for ‘Swallowing’ (Exclusively parenteral or enteral feeding). Loss of useful speech: Criterion was ALSFRS-R rating of 0 points for ‘Speech’ (Loss of useful speech). Abbreviations: ALSFRS-R: ALS functional rating scale-revised; BiPAP: bilevel positive airway pressure; FAS: full analysis set; E: edaravone group; P: placebo group.](/cms/asset/c1c10845-2338-4de3-b0f4-3ac6a9d44f9d/iafd_a_1364269_f0003_c.jpg)
Table 2. Adverse events (≥5%) and serious adverse events (more than one patient)Table Footnotea in the safety analysis set.
