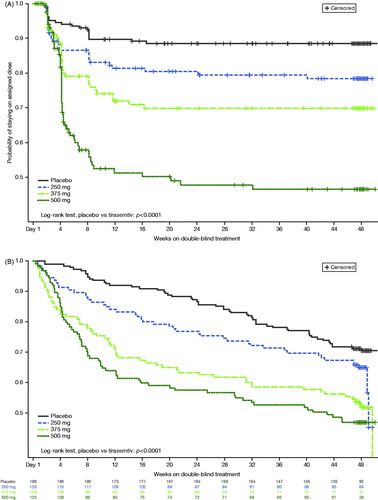
Figures & data
Table 1 Demographics and baseline disease characteristics.
Figure 1 Patient disposition. *One patient was randomized but did not receive study drug. AE: adverse event.
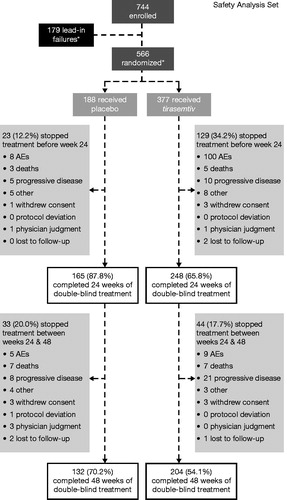
Table 2 Most common TEAEs during the 48-week, double-blind, placebo-controlled phase.
Table 3 Serious AEs occurring in >1% of participants during the 48-week double-blind phase.
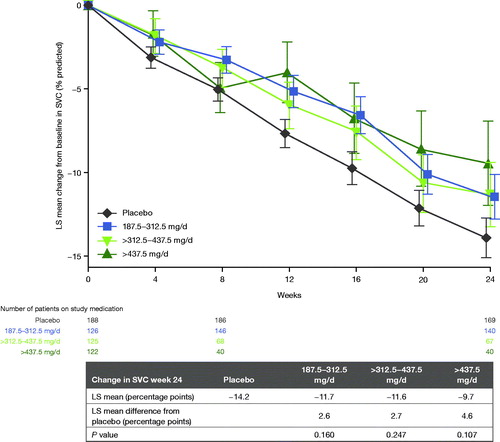
Table 4 Percent predicted SVC change from baseline to week 24 in PPS, mixed model for repeated measures.
Data availability statement
In collaboration with the ALS association, this study also included a biofluids collection at every visit. All demographic and efficacy data will be migrated to NeuroBANK, a database maintained by the NEALS Biorepository. Biofluids and phenotypic data will be available through application to the NEALS Biorepository Committee.