Figures & data
Table 1. Baseline ALSFRS-R scores for items 1, 3, 5, and 8 for patients in the placebo and reldesemtiv groups.
Figure 2. Probability of no new DME-PAP over time with reldesemtiv treatment compared with placebo. (A) Each dose of reldesemtiv, and (B) for all doses of reldesemtiv combined. Numbers of patients per group are indicated on top of the x-axis. bid: twice daily; DME-PAP: durable medical equipment prescribed and accepted by the patient.
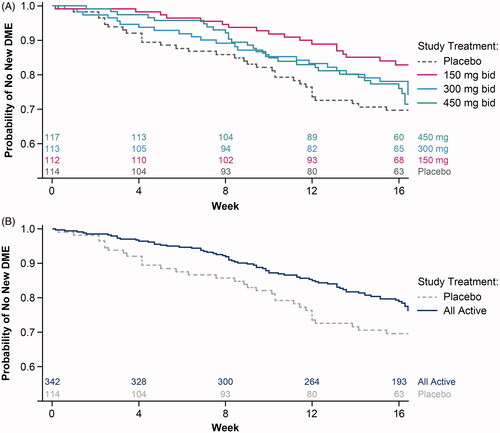
Table 2. Hazard ratiosa for reldesemtiv compared with placebo for at least one DME-PAP over the course of the trial.
Supplemental Material
Download MS Word (24.5 KB)Data availability statement
Data reported herein are part of a sponsor-led clinical development program that is ongoing, and thus complete datasets for the trial will not be made available with this report.