Figures & data
Figure 1 Sample characteristics. To evaluate the feasibility of remote digital assessment the ALSFRS-R, the total number of patients receiving treatment at the participating ALS centers was of interest. Therefore, an estimate of these patients was made – at the beginning of the observation, added by the patients who annually entered treatment at these centers (estimated multicenter cohort). Only a subpopulation of the multicenter cohort fulfilled the inclusion criteria (below) and contributed to this observational study (total study cohort). Among the total study cohort, two main sub-cohorts emerged: The first cohort included patients who allowed the secondary use of the existing data of clinical assessment of the ALSFRS-R (Clinic-ALSFRS-R cohort) but did not complete a rating via a computer or the mobile app. For this cohort, demographic and clinical data were collected and the Clinic-ALSFRS-R data were analyzed. The second main sub-cohort included patients who performed a remote digital assessment (Remote-ALSFRS-R cohort), either on a computer (Co-ALSFRS-R cohort) or on a mobile application (App-ALSFRS-R cohort), or on both. In a distinct cohort, other persons acted as patients’ deputy and realized the rating (PD-ALSFRS-R). Within the PD-ALSFRS-R cohort, no distinction was made between computer or mobile app use. Therefore, preferences of relatives regarding computer or app use were not analyzed. n: number of patients.
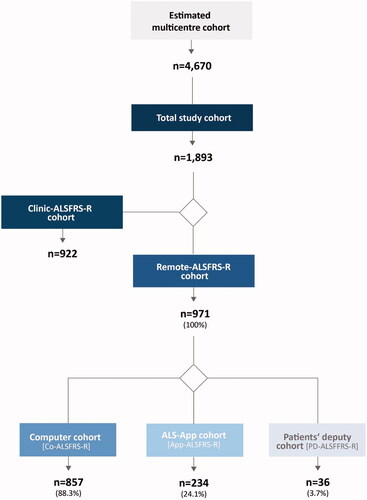
Table 1 Demographic and clinical characteristics of participants.
Table 2 Number of patients with assessments and assessments per patient per year.
Table 3 Results of ALSFRS-R ratings in relation to type of assessment.
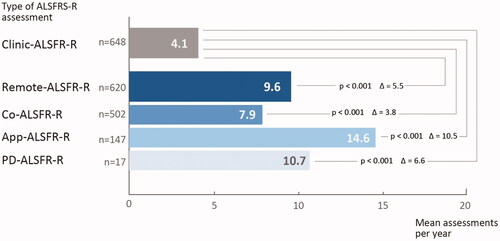
Figure 2 Frequency of ALSFRS-R ratings per year. Frequency of ALSFRS-R were obtained in the cohorts of clinical assessment of the ALSFRS-R (Clinic-ALSFRS-R) as compared to remote self-assessment using a computer (Co-ALSFRS-R) or mobile application (App-ALSFRS-R), or both. Significant differences were assessed by t-test. A p-value <0.05 was considered significant. Abbreviations: n: number of patients; ALSFRS-R: ALS Functional Ratings Scale Revised.
Supplemental Material
Download PDF (72.7 KB)Supplemental Material
Download PDF (223.5 KB)Supplemental Material
Download PDF (17 KB)Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
