Figures & data
Table 1 Major contributions to direct costs in the US (USD).
Figure 1 PRISMA flowcharts. ALS: amyotrophic lateral sclerosis; EMBASE: excerpta medica database; HCRU: healthcare resource use; HTA: health technology assessment; INAHTA: international network of agencies of health technology assessment; MEDLINE: medical literature analysis and retrieval system online; NHS-EED: national health service economic evaluation database; PRISMA: preferred reporting items for systematic reviews and meta-analyses; PRO: patient-reported outcome; QoL: quality of life; RCT: randomized controlled trial; SLR: systematic literature review; TLR: targeted literature review; US: United States. For the epidemiology TLR, registry/database studies were prioritized for extraction; data from small single-center studies and other less-robust study types were deprioritised. Flowcharts include all articles identified in the TLR, including some studies relevant to outcomes not presented in this publication. For a complete list of outcomes and eligibility criteria applicable to the review, see Table S6.
Figure 2 Summary of study designs. HCRU: healthcare resource use; MDA: muscular dystrophy association. *These studies were included in multiple evidence streams. **All articles by Mehta et al. are part of the ongoing CDC National ALS Registry surveillance project and are therefore considered as one study. MDA (Citation9) and Larkindale et al. (Citation7) were identified from high quality SLRs (Citation6, Citation12).
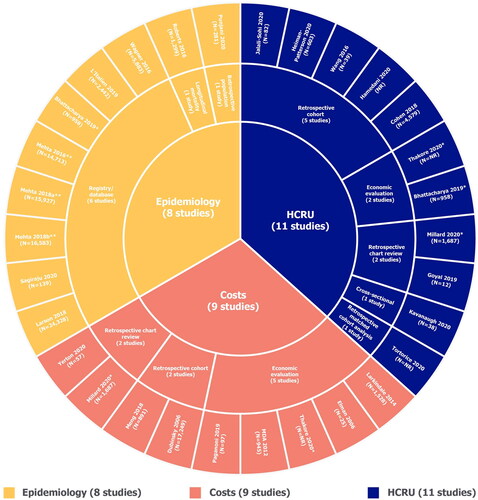
Figure 3 ALS prevalence, including stratification by sex, race/ethnicity and age. ALS: amyotrophic lateral sclerosis. Point prevalence refers to the proportion of a population with ALS at a specific time point. Period prevalence refers to the proportion of ALS cases at any point during the period of interest. Refer to Table S7 for details regarding design of each study (e.g. retrospective cohort, database/registry study). Both Punjani 2020 and Wagner 2016 deliberately over-represented racial/ethnic minorities in the study catchment areas. A) Overall unadjusted prevalence reported for Bhattacharya 2019; direct age-adjusted point prevalence for 2011 = 9.7 per 100,000 population; direct age-adjusted period prevalence for 2007–2011 = 15.1 per 100,000 population.
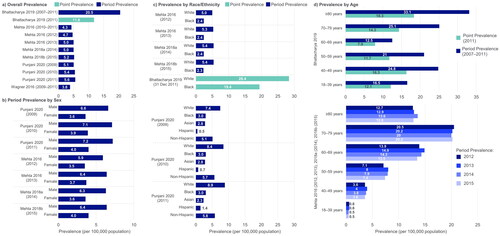
Figure 4 Estimated annual national costs. MDA: muscular dystrophy association; USD: United States dollars. Each study used three different prevalence estimates for the US to calculate annual costs. Societal perspective, considering all costs to society, including costs to patients, government and healthcare payers, sometimes reporting these separately. MDA 2012 (Citation9): 3396 Cases; Alčaz et al. 1996 (Citation47): 16,055 cases; Orphanet 2011 (Citation48): 22,847 cases; Worms 2011 (Citation49); Larkindale et al. 2014 (Citation7): 16,055 cases; Orphanet 2021 (Citation50): 6792 and 4104 cases; Wagner et al. 2012 (Citation51).
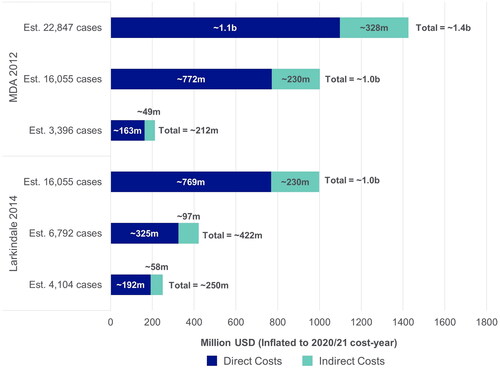
Figure 5 Total overall, direct and indirect costs (USD, PPPY). MDA: muscular dystrophy association; PPPY: per patient per year; USD; United States dollars. Each bubble represents one study and bubble size represents the study sample size. Meng 2018 has two bubbles as total direct costs were reported separately for different insurance cohorts; total annual direct costs were extrapolated from monthly costs by multiplying by 12; the cost used was “average costs for months post-diagnosis” but different monthly costs were reported for the month of diagnosis and the month of death separately; Meng 2018 was conducted from the payer perspective and the costs reflect the amount paid by insurance companies. Furthermore, only medical costs were considered therefore the costs may not be reflective of overall total direct costs. Annual total direct and total indirect costs in Thakore 2020 were extrapolated from monthly costs by multiplying by 12 and using the average cost over four different disease stages; for total direct costs, only recurring costs were included; for total indirect costs, the human capital approach was used in that absenteeism was considered as a recurring cost beyond death; total overall costs were not reported for Thakore 2020, therefore were calculated by summing total direct and total indirect costs. Direct costs such include hospitalization costs, treatment costs, and non-hospital care costs; indirect costs include productivity loss (including caregiver productivity loss), home adaptations, and travel costs. Figure legend indicates what perspective the study used: Societal perspective, considering all costs to society, including costs to patients, government and healthcare payers, sometimes reporting these separately; Payer perspective, considering only costs to healthcare providers or insurance companies.
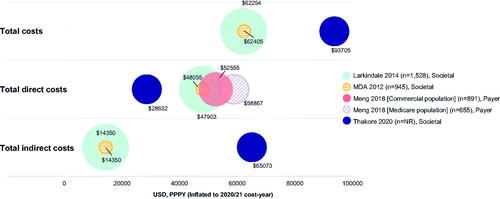
Table 2 Costs associated with different disease stages/milestones (USD).
Supplemental Material
Download MS Word (107.9 KB)Data availability statement
The data supporting the conclusions of this article are included within the article and its additional files. Full search results available on reasonable request.
