Figures & data
Table 1. Characteristics of men in Prostate Cancer data Base Sweden (PCBaSe) RAPID with prostate cancer registered between 1 January 1998 and 31 December 2014, in the National Prostate Cancer Register and residing in Sweden on 1 July 2015.
Figure 1. Timeline of the study period in the Prostate Cancer data Base Sweden (PCBaSe) RAPID. NPCR: National Prostate Cancer Register; PDR: Prescribed Drug Register; NOVA: novel antiandrogen; FU: follow-up; TPR: Total Population Register (vital status). The grey rectangle represents the study period for filled prescriptions for NOVAs in the PDR. A–E illustrate the different periods during which a patient could be at risk before death or censoring: patient A was at risk during the whole study period; patients B and C died during the study period; patient D died before 1 November 2015 and is not included in the analysis of users vs non-users; patient E died before 1 July 2015 and is not included in the study.
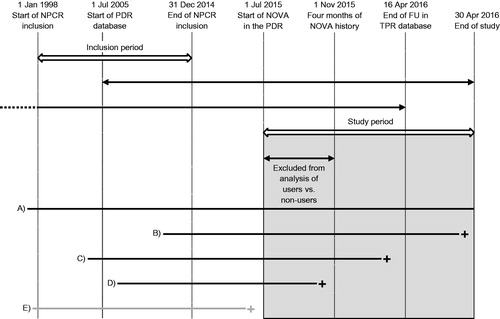
Table 2. Characteristics of men with a high probability of castration-resistant prostate cancer in the Prostate Cancer data Base Sweden (PCBaSe) RAPID, registered in the National Prostate Cancer Register between 1 January 1998 and 31 December 2014 with distant metastases at the date of diagnosis, on gonadotropin-releasing hormone (GnRH) therapy on 31 December 2014 and residing in Sweden on 1 November 2015.
Table 3. Odds ratios (ORs) and 95% confidence intervals (CIs) for receipt of a novel antiandrogen (NOVA).
Figure 2. Proportion of men with prostate cancer (PCa) diagnosed in 2014 who had a prescription filled for (a) a novel antiandrogen (NOVA), (b) abiraterone or (c) enzalutamide, between 1 July 2015 and 30 April 2016 per 1000 deaths from PCa in each county in 2013–2015.
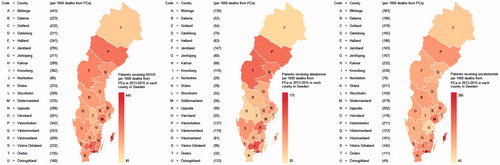
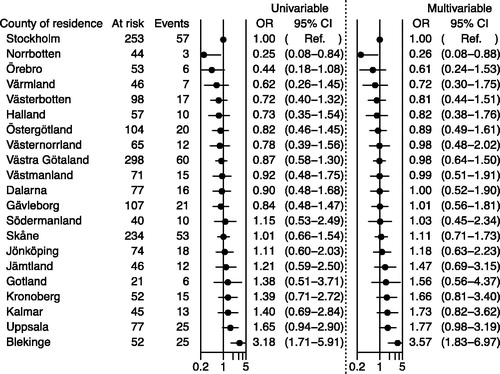
Figure 3. Odds ratios (ORs) and 95% confidence intervals (CIs) for receipt of novel antiandrogens (NOVAs) according to county of residence: logistic regression model including men registered in the National Prostate Cancer Register (NPCR) between 1 January 1998 and 31 December 2014 with metastatic cancer, on gonadotropin-releasing hormone (GnRH) agonist on 31 December 2014. All men included in the figure were at risk on 1 November 2015. Filling of a prescription for a novel antiandrogen (NOVA), abiraterone or enzalutamide, was determined between 1 July 2015 and 15 April 2016. A logarithmic scale (base 10) was used. The adjusted model includes year of diagnosis, age, comorbidity, marital status and educational level.