Figures & data
Table 1. Key differences in patient characteristics at baseline in the registry study conducted at Skåne University Hospital and randomised clinical trials of enzalutamide in patients with mCRPC [Citation5,Citation6,Citation14,Citation15].
Figure 1. Kaplan–Meier plot of overall median survival from enzalutamide initiation to death from any cause in post-chemotherapy patients with mCRPC and prior docetaxel treatment. HR: hazard ratio; mCRPC: metastatic castration-resistant prostate cancer. aHR from time-to-event analysis.
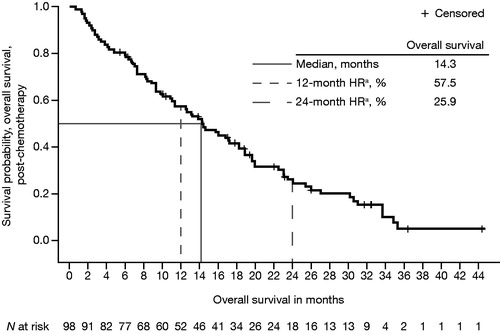
Figure 2. Kaplan–Meier plot of median treatment duration, from enzalutamide initiation to all-cause treatment discontinuation in pre-chemotherapy patients with mCRPC. HR: hazard ratio; mCRPC: metastatic castration-resistant prostate cancer. aHR from time-to-event analysis.
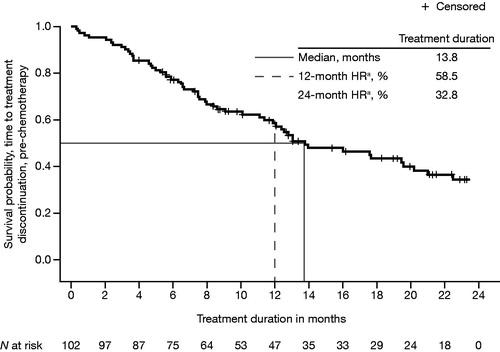
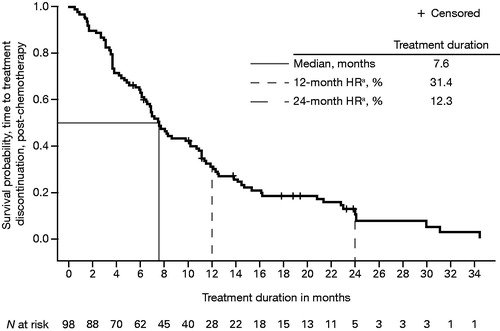
Figure 3. Kaplan–Meier plot of median treatment duration, from enzalutamide initiation to all-cause treatment discontinuation in post-chemotherapy patients with mCRPC. HR: hazard ratio; mCRPC: metastatic castration-resistant prostate cancer. aHR from time-to-event analysis.
Alghazali_et_al_supplemental_appendix.docx
Download MS Word (42.1 KB)Data availability
Access to anonymised individual participant-level data will not be provided for this trial, as it meets one or more of the exceptions described on www.clinicalstudydatarequest.com under ‘Sponsor Specific Details for Astellas’. The ethical approval limits the access to data.
