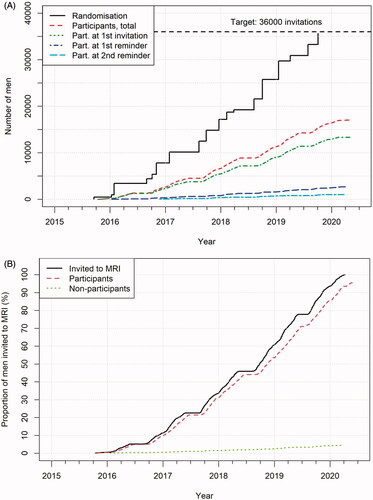
Figures & data
Table 1. Primary and secondary outcomes in Göteborg-2 trial.
Table 2. Definitions of clinically significant cancer.
Table 3. Eligibility criteria.
Figure 1. Study Schema of the Göteborg-2 trial. Figure 1 shows the study layout of the Göteborg-2 trial. MRI interpretation is performed according to Prostate Imaging-Reporting and Data System (PI-RADS), v.2.1. MRI +: positive MRI defined as PI-RADS 3, 4 or 5. MRI−: negative MRI defined as PI-RADS 1 or 2. *All men with PSA ≥ 10.0 ng/mL are recommended 12-core systematic TRUS biopsy plus additional targeted biopsy if positive MRI. **All men with an MRI showing PI-RADS 5 are recommended 12-core systematic TRUS biopsy.
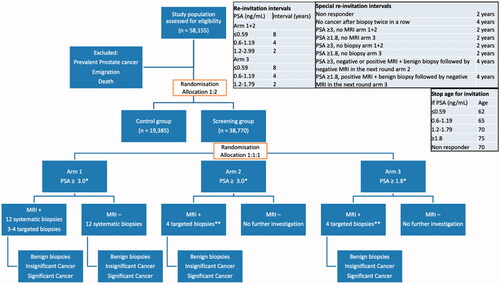
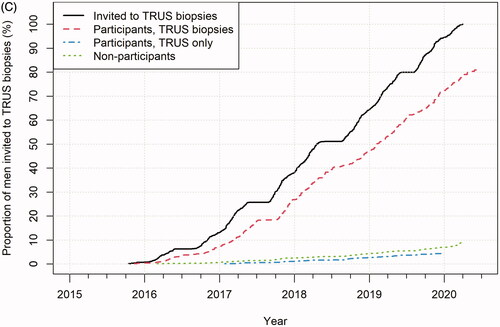
Figure 3. (A) Reasons for MRI not performed or MRI of non-diagnostic quality.Of the planned MR exams, 6% were not performed (due to participant declining or having a medical contraindication) or of non-diagnostic quality (mainly due to hip-prostheses or other osteosynthesis material distorting the diffusion-weighted sequence but also due to claustrophobia rendering incomplete examinations). (B) Reasons for TRUS biopsies not performed. Of the planned TRUS biopsies, 15% were not performed.
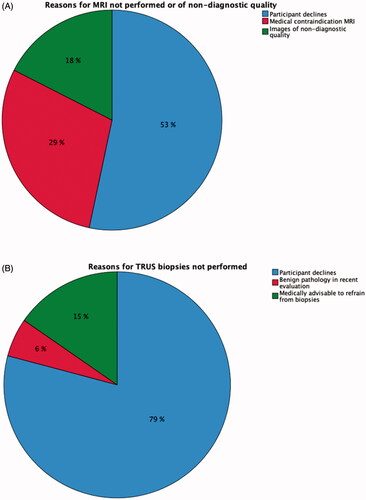
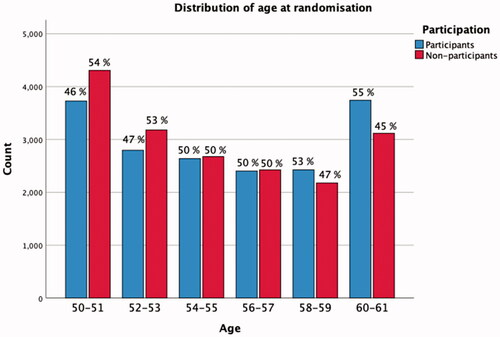
Figure 4. Age distribution at randomisation. Due to time delay between the identification of the first random sample from the Total Population Register and the randomisation to control or screening group, some men turn 61. They make 1% of the randomised men up-until 31 December 2019.