Figures & data
Figure 1. Concentration response curve for indomethacin-induced loss of barrier function in the MKN-28 cell line. (A) Indomethacin (100 µM-1 mM) decreased TER in a concentration-dependent manner. Indomethacin concentrations of 500 µM, 700 µM, and 1 mM reduced the TER significantly at 24-hours post-treatment. *p < 0.05. (B) Indomethacin (100 µM-1 mM) increased the FITC dextran flux in a concentration dependent manner. Indomethacin concentrations of 500 µM, 700 µM, and 1 mM increased FITC dextran flux significantly at 24-hours post-treatment. *p < 0.05. (C) Examination of indomethacin-induced cell toxicity by LDH release assay. Indomethacin concentrations of 700 µM, and 1 mM caused significant increase in LDH release 24-hours post-treatment, whreas there was no increase in LDH in cells exposed to 500 µM over the same time period. *p < 0.05. These data are represented as the average of more than 3 identically treated monolayers, from 3 independent experiments.
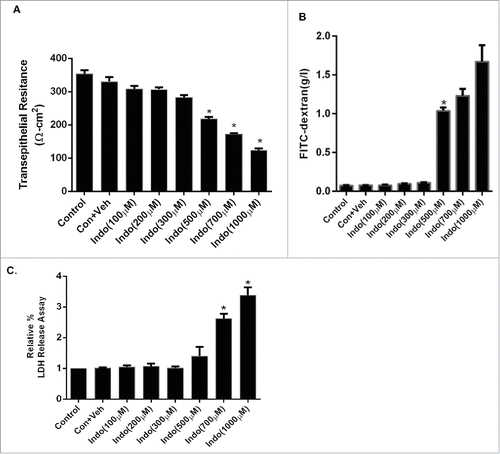
Figure 2. Effect of MAPK inhibition on indomethacin-induced changes in the epithelial barrier in MKN-28 cells. (A) Indomethacin (500 μM) reduced the TER significantly at 24-hours post treatment, while inhibition of p38 MAPK and JNK significantly attenuated the drop in TER caused by indomethacin (*p < 0.005). (B) Indomethacin (500 µM) caused a significant increase in the paracellular permeability of dextran (4KD) in MKN-28 cells (24-hours post exposure), while consistent with effect on TER, p38 MAPK and JNK inhibition attenuated the indomethacin-induced increase in paracellular permeability (#p < 0.05 vs Indo alone). The data are represented as the average of more than 3 identically treated monolayers, from 3 independent experiments.
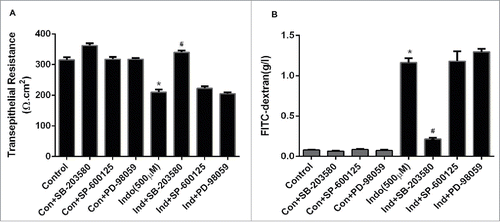
Figure 3. Evaluation of MAPK phosphorylation in MKN-28 cells in the presence of indomethacin. (A) Indomethacin-induced phosphorylation of p38 MAPK was blocked by pretreatment with the p38 MAPK inhibitor SB-203580. Similarly the indomethacin induced phosphorylation of JNK and ERK was also blocked by SP-600125 and PD-98059 respectively. Indomethacin-treated cells, though, showed phosphorylation of ERK, it was not significantly different from the control cells. (*p < 0.05 vs. control). (B) Relative densitometry of phosphorylated p38 MAPK as compared to total p38 MAPK. The data are represented as the average of more than 3 identically treated monolayers from 3 independent experiments. The data are presented the same way for the other MAPK analyses in parts C and D. (C) Relative densitometry of phosphorylated JNK as compared to total JNK. (D) Relative densitometry of phosphorylated ERK as compared to total ERK.
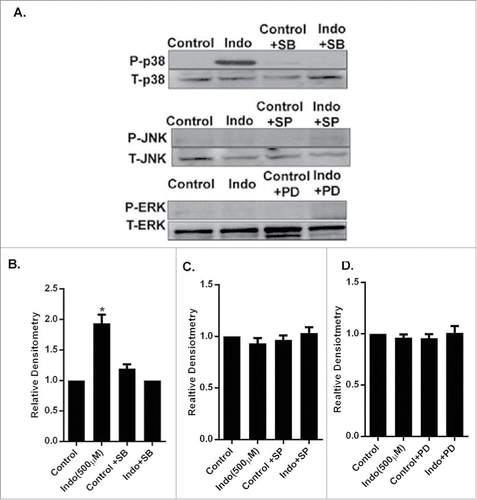
Figure 4. Indomethacin causes a decrease in occludin via activation of p38 MAPK. (A) Indomethacin exposure for 24 hours markedly reduced expression of occludin but not claudin-2, claudin-4 or ZO-1. Inhibition of p38 MAPK with SB-200358 attenuated the loss of expression of occludin caused by indomethacin. (B) Relative densitometry of occludin to control levels, confirming the significant decrease in occludin expression in the presence of indomethacin alone. Inhibition of p38 MAPK with SB-203580 significantly attenuated the reduced expression of occludin. (*p < 0.05 vs. control and Indo + SB). The data are represented as the average of more than 3 identically treated monolayers from 3 independent experiments. (C) MKN-28 cells after pretreatment with MAPK inhibitors for 1 hour were exposed to indomethacin (500 μM) for 24 hours. Cells were fixed and stained for occludin (green fluorescence) and examined by confocal microscopy. Immunolocalization of occludin at the tight junctions (arrow in control) was found to be disrupted in indomethacin exposed cells (500 µM) (arrow) while inhibition of p38 MAPK with SB-203580 prevented the loss of occludin at the TJs (arrow) 200X. The images are representative of more than 3 identically treated monolayers from 3 independent experiments.
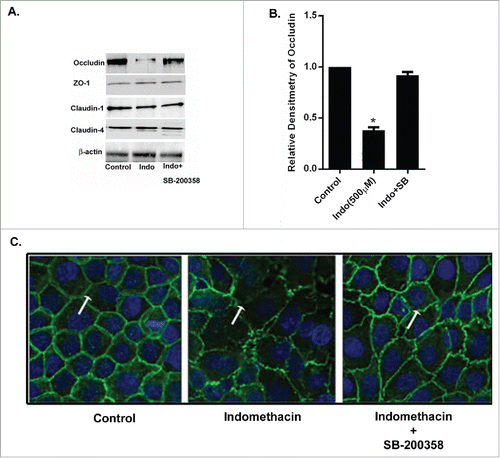
Figure 5. Effect of p38 siRNA on occludin in indomethacin-treated MKN-28 cells. (A) SiRNA of p38 MAPK prevented the decrease in transepithelial resistance caused by indomethacin (500 µM) when compared to control. Non-specific SiRNA did not have an appreciable effect on control cells The data are represented as the average of more than 3 identically treated monolayers from 3 independent experiments. (*p < 0.001 vs. control and Ind+siP38). (B) SiRNA of p38 MAPK attenuated the increase in inulin flux caused by indomethacin (500 µM) across the MKN-28 cells when compared to control. The data were generated in the same way as panel A. (*p < 0.001 vs. control). (C) Indomethacin caused a decrease in tight junction occludin protein expression. Knocking-down p38 MAPK by siRNA transfection prevented the indomethacin-induced decrease in occludin expression as assessed by western analyses. This blot is representative of the data shown in panels D and E. (D) Relative densitometry for phosphorylated p38 MAPK in the presence of indomethacin, and in cells transfected with siRNA for p38 MAPK. The data is represented as the average of more than 3 identically treated monolayers, from 3 independent experiments. (E) Relative densitometry of occludin in cells treated with indomethacin or indomethacin in cells transfected with siRNA for p38MAPK. The data are shown in the same way as panel D.
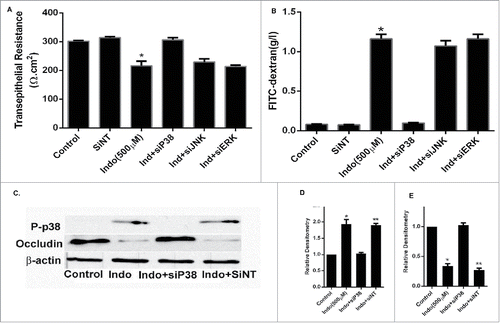
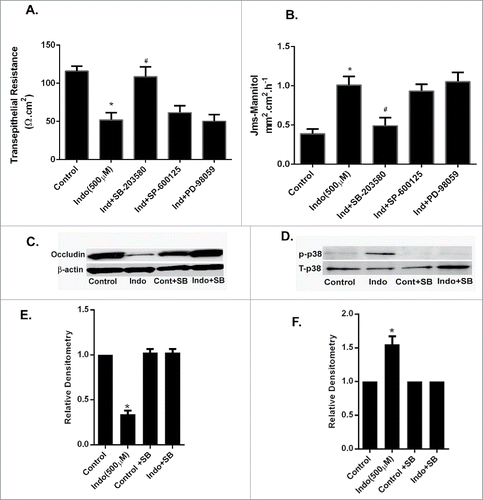
Figure 6. Effect of MAPK inhibition on indomethacin-induced alterations in gastric barrier function. (A) The gastric mucosa from wild type C57/BL-6 mice (n = 6) was mounted on Ussing chambers. Indomethacin (500 µM) reduced the TER significantly (*p < 0.05, compared to control cells, #p < 0.05 compared to Indo+SB) while pretreatment with p38 MAPK and JNK inhibitors prevented the drop in TER caused by indomethacin. (B). In the Ussing chamber experiments, as in A, indomethacin caused a significant increase in paracellular permeability of mannitol in the gastric mucosa compared to untreated tissues (*p < 0.05, compared to control cells, #p < 0.05 compared to Indo+SB). In the gastric mucosa pretreated with p38 MAPK and JNK inhibitors, the mannitol permeability of indomethacin-treated tissues was comparable to that of control gastric mucosa. (C) Gastric tissues from Ussing chamber experiments were collected and subjected to western analyses. Indomethacin exposure for 90-minutes caused reductions in expression of occludin. (D) Western analysis of gastric tissues collected as in C showed an increase in the expression of phosphorylated p39 MAPK in the presence of indomethacin alone, which was markedly reduced by additional treatment with the p38 MAPK inhibitor SB-203580. (E) Relative densitometry of the n = 6 tissues western blotted for occludin expression (representative blot shown in C). Note the significant reduction in occludin expression caused by treatment with indomethacin (500 μM), which was restored by pre-treatment with the p38 MAPK inhibitor SB-203580 (*p < 0.05 vs. all other treatment groups). (F) Relative densitometry of the n = 6 tissues protein gel blotted for p38MAPK expression (representative blot shown in D). Note the significant increase in phosphorylated p38 MAPK in the presence of indomethacin (500 μM), which was reduced by pre-treatment with the p38 MAPK inhibitor (*p < 0.05 vs. all other treatment groups).