Figures & data
Figure 1. 3D-model of the EC1-domain of LI-cadherin. (A) A model of the EC1-domain of LI-cadherin. The model is based on an energy minimisation of the primary sequence of LI-cadherin EC1 initiated by a template based on X-ray crystallography of E-cadherin EC1. Based on comparison with other cadherins (VE-cadherin, N-cadherin and E-cadherin) the loop between the β-sheets β6 and β7 (indicated by the arrow) was identified to be one binding site for LI-cadherin trans-interaction and an inhibitory peptide of the sequence VAALD was identified there. The aspartic acid of this peptide in the loop between β6 and β7 is indicated. (B) A model of the trans-interaction of 2 LI-cadherins. The orientation of the lower EC1 is identical to the single EC1 shown in (A). The loop between β6 and β7 interacts with corresponding amino acids in the vicinity of β4. This binding region close to β4 is indicated in (A) by the arrow-head.
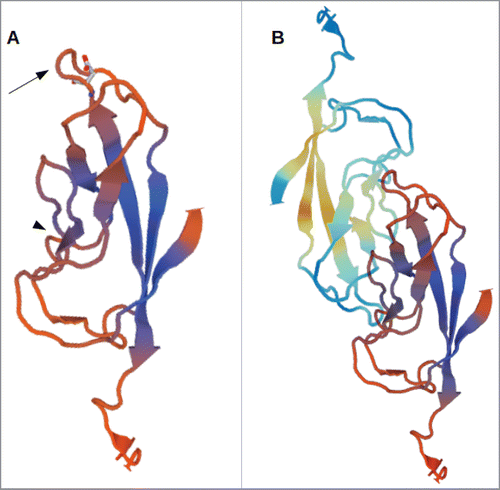
Figure 2. Affinity shift chromatography of LI-cadherin in the presence and absence of VAALD. (A) Dot-blot of the fractions from the eluation of a LI-cadherin-Fc column. While there is a significant delay under control conditions of the eluation of free LI-cadherin-Fc, in the presence of VAALD or in the absence of Ca2+, the LI-cadherin is eluated directly without specific interaction. A quantification is shown in (B) and (C).
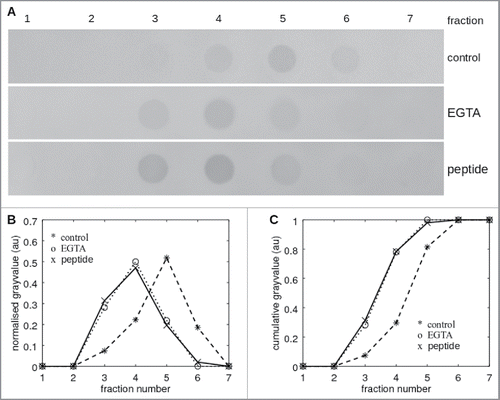
Figure 3. Staining of LI-cadherin in CACO2-cells with LI-cadherin specific antibodies (A) or with the fluorescently labeled peptide VAALD (B). Clearly the LI-cadherin is mainly found at cell-cell-boarders. Clearly there is a localization of the peptide and the antibody along the cell boarders. It has to be emphasized that the cells for the peptide staining were not permeabilised to see whether or not the peptide can enter the lateral intercellular cleft for our transport measurements.
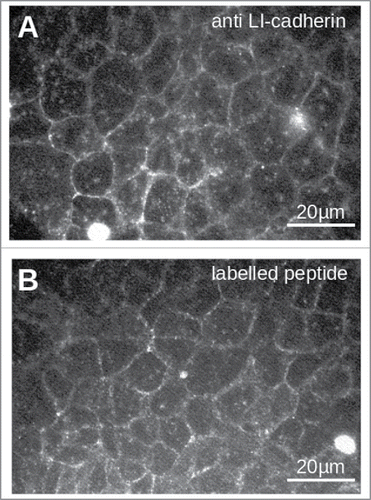
Figure 4. Typical flux measurements under different osmotic conditions in the presence and absence of the inhibitory peptide. The flux was determined indirectly by measuring the mass difference of the insert over time. While normally the cells can cope with a rather high osmotic gradient for water into the lumen, the inhibitory peptide impairs this ability. Under isotonic conditions the peptide has no visible effect. The panels correspond to 3 independent measurements on different trans well filters.
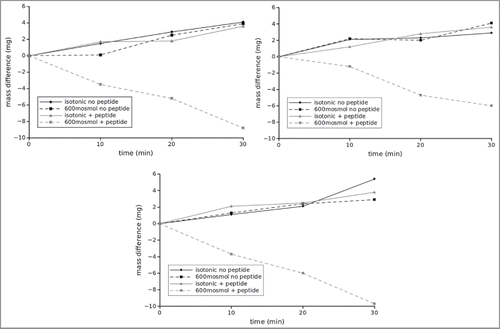
Figure 5. Analysis of flux measurements under different osmotic conditions in the presence and absence of the inhibitory peptide. Here values to the right correspond to flux out of the gut into the interstitium while columns to the left indicate flux into the lumen. All columns correspond to at least 6 independent measurements, i.e., 6 different inserts. Asterisks indicate statistical significant differences (2 sided unpaired t-test, p < 0.01).
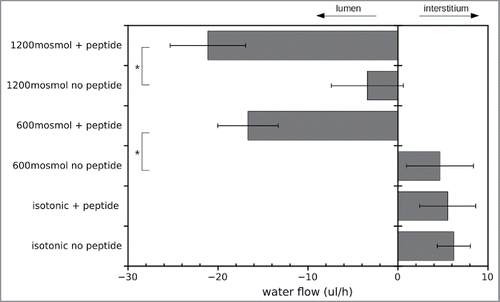
Figure 6. Transmission electron microscopic images of CACO-cells under different osmotic conditions in the presence and absence of the inhibitory peptide. (A) Overview of the intercellular cleft in the presence of the inhibitory peptide under hypertonic conditions. Apically the microvilli could be seen. The basal membrane on the transwell filter is indicated by arrows. The intercellular cleft (indicated by arrowheads) clearly becomes more and more impaired from apical to basal. Irregular widening of the cleft was observed. (B) Apical section of CACO-cells in the vicinity of the junctional complex under hypertonic conditions. Note no obvious morphological change of the junctional complex. (C) CACO-cells in the absence of the inhibitory peptide under hypertonic conditions. The intercellular cleft (arrow heads) is constantly narrow.
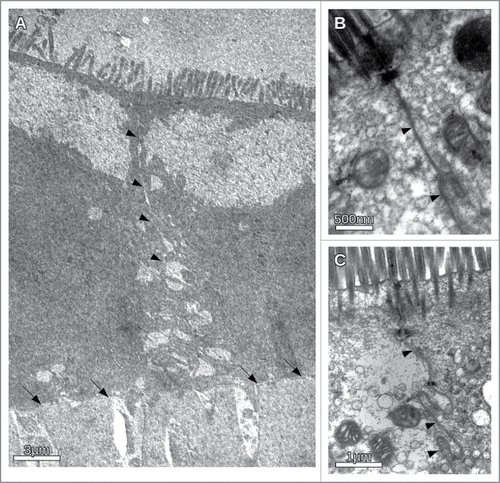
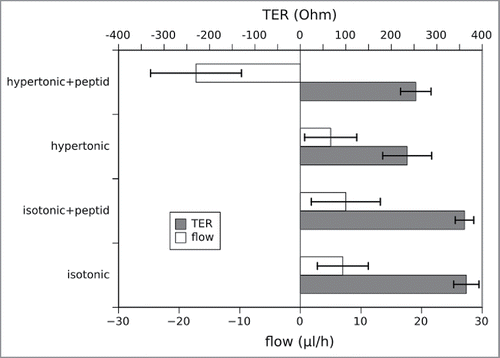
Figure 7. Simultaneous measurement of the water flux and the transepithelial resistance (TER) under different osmotic conditions in the presence and absence of the inhibitory peptide. The data represent mean values of 3 independent measurements, errorbars show the standard deviation. While the flux behaves as in the previous measurements in , the TER is not affected by the presence or absence of the peptide (2 sided unpaired t-test, p < 0.01). Of course the TER differs dependent on the osmotic conditions and is lower in the hypertonic medium due to the higher ion concentration.