Figures & data
Figure 1. Confocal micrographs of the stomach mucosa stained for tight junction associated claudin-4 and basolateral membrane-associated claudin-18. Note the absence of green fluorescence signal at apical tight junctions in the surface of tissues stained for claudin-4 but the strong signal at apical tight junctions of epithelial cells in the neck and base (arrows). In contrast, claudin-18 is highly concentrated at the basolateral membrane of all epithelial cells (arrowheads) in the stomach mucosa with particularly robust expression in surface epithelial cells. MM, muscularis mucosa.
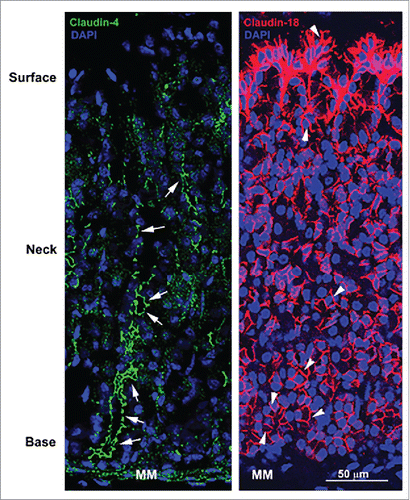
Table 1. Tissues and cultured cells in which tight junction proteins are found outside the apical tight junction complex.Footnote*
Figure 2. Schematic diagram of potential functions for basolateral claudin molecules. (A) Basolateral claudins may function in the trafficking of endosomal vesicles (red circles) from the baolateral membrane to tight junctions for rapid remodeling. (B) Basolateral claudins may function as accessory pores to facilitate further discretion of ion transport at tight junctions (dotted arrow) and/or create signaling hubs via interaction with claudin/claudin complexes and the action cytoskeleton. EpCAM, epithelial cell adhesion molecule; TGN, trans-Golgi network.
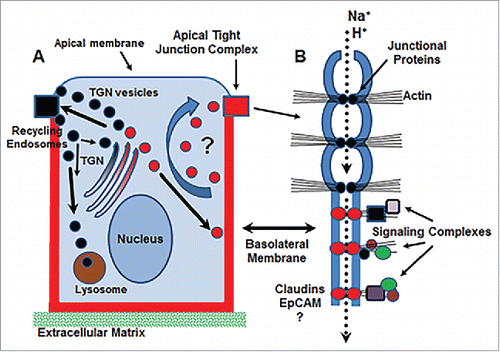
Figure 3. Schematic diagram of the cell/extracellular matrix interface in epithelial cells. Claudin molecules interact with integrin -α and -β subunits within the focal adhesion complex, which provides and anchor for cells to the extracellular matrix. The presence of claudins results in MAPK signaling through the focal adhesion kinase (FAK)/Src/p 130cas complex.
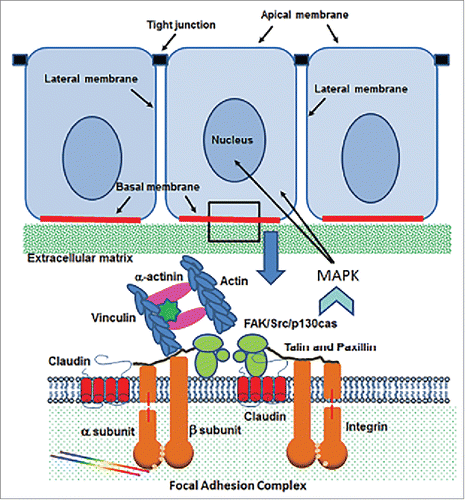
Table 2. Putative nuclear localization sequence (NLS) for mouse claudins determined from cNLS Mapper at http://nls-mapper.iab.keio.ac.jp/cgi-bin/NLS_Mapper_form.cgi
