Figures & data
Figure 1. Topology and domain structure of TRPC6. Each of the 4 TRPC6 monomers within a TRPC6 homotetramer contains 6 transmembrane domains with the pore region (LFW, pore motif) between domains 5 and 6. Structural domains for channel assembly and protein interaction sites are located on the cytoplasmic N and C termini. Figure adapted from ref. Citation2 with updates.
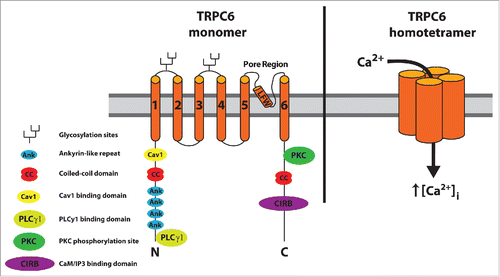
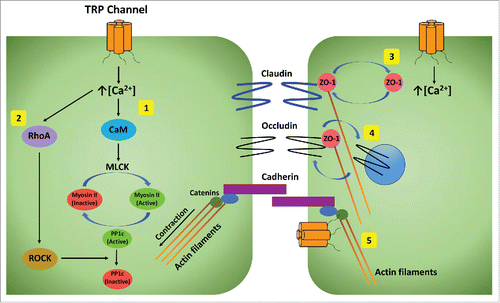
Figure 2. Effector pathways from TRP channels that regulate barrier function. The ways that TRP channels influence barrier function include promoting intracellular tension by actin-myosin contraction mediated by calcium induced (1) activation of myosin light chain kinase (MLCK) through calmodulin (CaM), (2) RhoA activation of Rho kinase (ROCK) to inhibit inactivation of myosin phosphatase PP1c, (3) altering distribution of ZO-1 between tight junctions and cytoplasm, (4) endocytosis of occluding from tight junctions, and (5) direct interaction with catenins to influence their association with actin filaments.