Figures & data
Table 1. Antibodies used in Western blotting and immunofluorescence staining.
Table 2. Real-time PCR primers used in this study.
Figure 1. Alterations in tight and adherens junction proteins detected by Western blot TJ and AJ proteins such as ZO-1, occludin, claudin-2, claudin-4, claudin-12, β-catenin and VE-cadherin were significantly decreased in VDR-/- mice, but claudin-10 showed higher protein levels in VDR-/- mouse lung tissues than in WT mouse lung tissues (n = 3–5; * P < 0.05; ** P < 0.01).
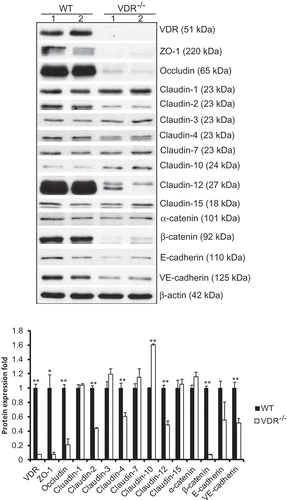
Figure 2. Alterations in tight and adherens junction mRNA expression detected by q-PCR. The mRNA expression of most TJ and AJ components showed a decreasing trend in VDR-/- mouse lung tissues. There were significant differences in claudin-2, claudin-4, claudin-10, claudin-12 and claudin-15 mRNA levels between VDR-/- and WT mouse lung tissues (n = 3–5; * P < 0.05; ** P < 0.01).
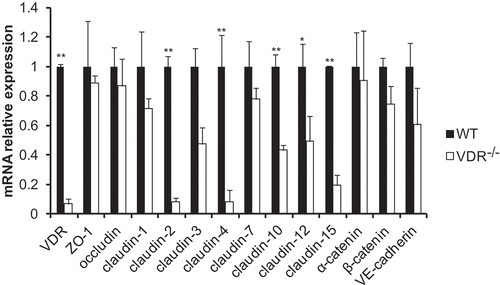
Figure 3. Distribution of tight and adherens junction proteins in mouse lung tissues. VDR protein was located in the cytoplasm of bronchial epithelial cells in only WT mice, with nearly no expression in VDR-/- mice. ZO-1 and occludin were located at the cell membrane of alveolar epithelial cells and bronchial epithelium cells. Positive signals for claudin-2 was detected in only the cytoplasm of bronchial epithelium cells, and more positive signals were observed in WT mouse lung tissues. Claudin-3 and claudin-4 could be observed at the cell membrane of bronchial epithelium cells; moreover, claudin-4 levels were significantly decreased in VDR-/- mouse lung tissues. Claudin-10 protein expression was found at the cell membrane and in the cytoplasm of bronchial epithelium cells. Positive signals for claudin-12 were mostly located in the cytoplasm of bronchial epithelium cells, and stronger positive signals could be detected in lung tissues from WT mice than in that from VDR-/- mice. Positive signals for claudin-15 protein were detected in the cell membrane of mouse bronchial epithelium cells. AJs proteins, E-cadherin and β-catenin, had the same expression pattern, with primary localization at the cell membrane of alveolar epithelial cells and bronchial epithelium cells.
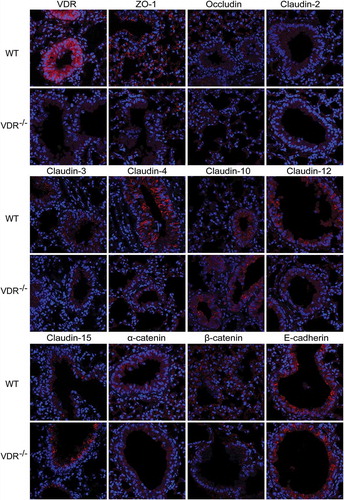
Figure 4. Colocalization of occludin and claudin-10 in mouse lung tissues. Positive signals for claudin-10 protein were red, those for occludin protein were green, and colocalization of occludin with a claudin protein was yellow. Claudin-10 protein had higher expression in VDR-/- mouse lung tissues than in WT mouse lung tissues. On the contrary, occludin protein showed low levels of expression in the VDR-/- mouse lung tissues.
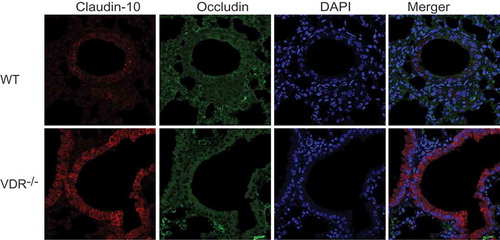
Figure 5. The expression of claudin-18 in lung tissues with or without VDR. (A) Claudin-18 expression at the mRNA level tissues was significantly reduced in VDR-/- lung, tested by real-time PCR. (B) Claudin-18 protein in lung tissues was detected by Western blots. (C) Claudin-18 was observed at the cell membrane of bronchial epithelium cells in mouse lung tissues by Immunofluorescence staining. Claudin-18 was decreased in VDR-/- mice. (n = 3; * P < 0.05; ** P < 0.01).
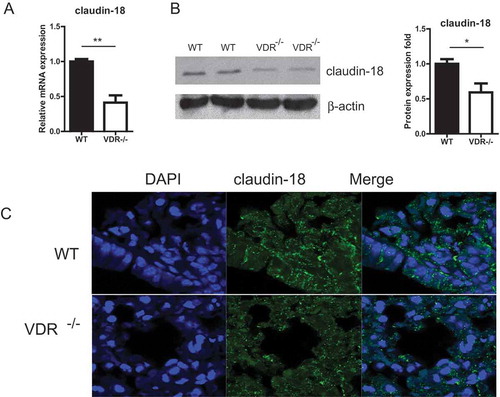
Table 3. Histopathological scores of mice with wild type VDR and VDR-/-.
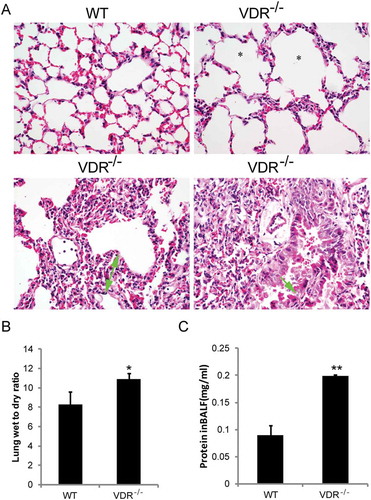
Figure 6. Histopathological alterations and increased lung permeability in VDR-/- mice. (A) Emphysema (star showed), chronic inflammatory cell infiltration (white arrowhead), lung septa more than two times broadening (green arrow with start and end) and bronchial epithelium detachment (green arrow) in VDR-/- mice lung tissues, compared with the wild type mice (magnification, × 400). (B) The lung wet-to-dry weight ratio. (C) Total protein concentration in the BALF (n = 3–5; * P < 0.05; ** P < 0.01).