Figures & data
Table 1. Primary and secondary antibodies used for this study are shown including the concentrations in Western blot (WB) experiments and in immunostaining (IS).
Figure 1. Plakophilin2 and Plakophilin3 are expressed in human intestinal tissue.
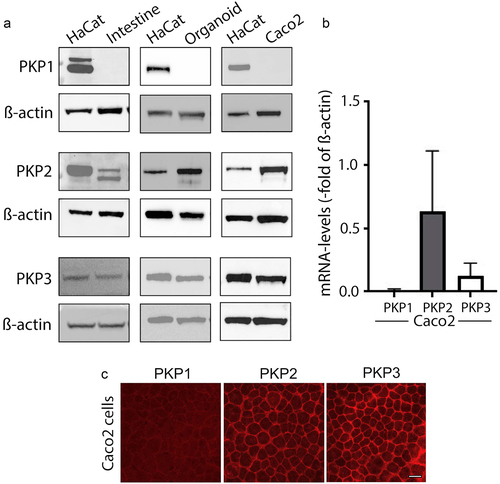
Figure 2. Effects following knockdown of PKP2, 3 or PKP2/3.
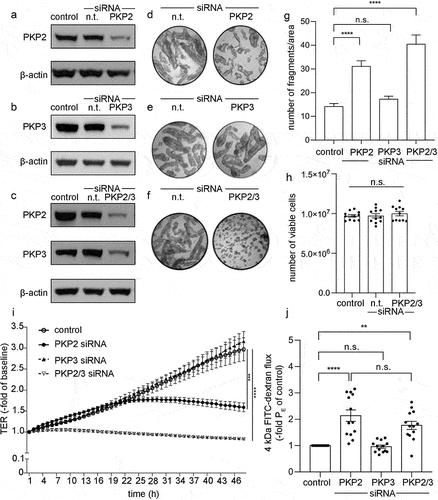
Figure 3. Effects of PKP knockdown on desmosomal proteins in Caco2 cells
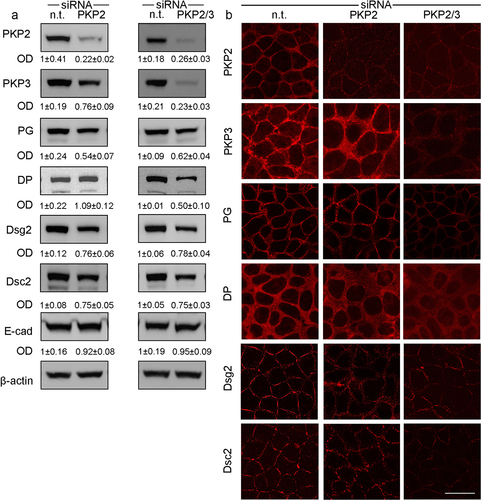
Figure 4. Protein kinase C is involved in barrier-compromising effects by PKP2.
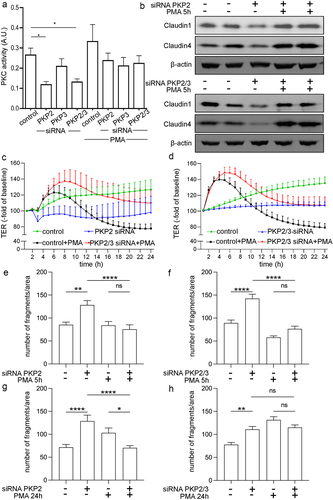
Figure 5. PKP2 interacts with protein kinase C.
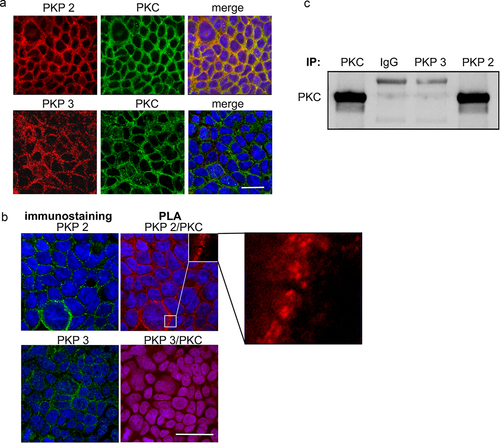
Figure 6. PKP2 and PKP3 overexpression had no effect on barrier functions.
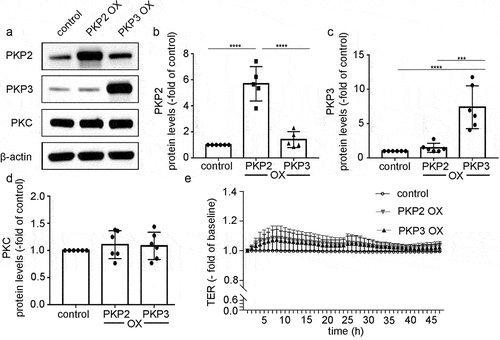
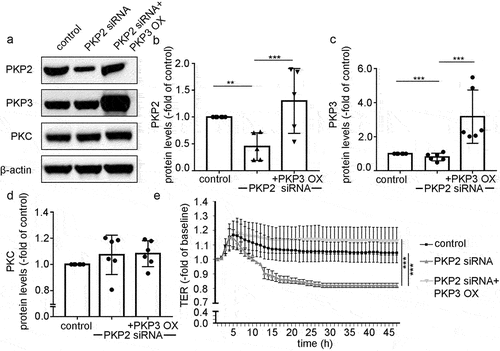
Figure 7. PKP3 overexpression in Caco2 cells attenuated the effect of PKP2 siRNA.