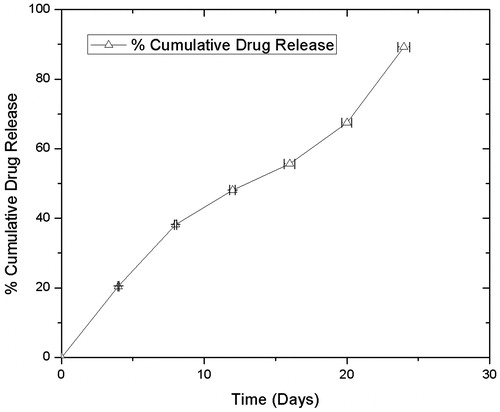
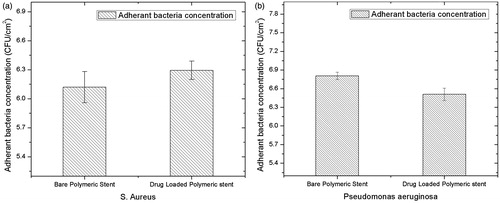
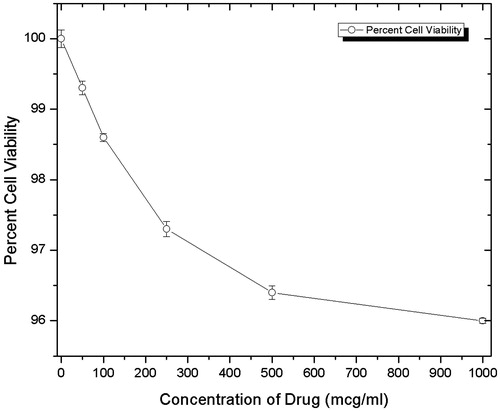
Figures & data
Table 1. Optimization of bare polymeric stent.
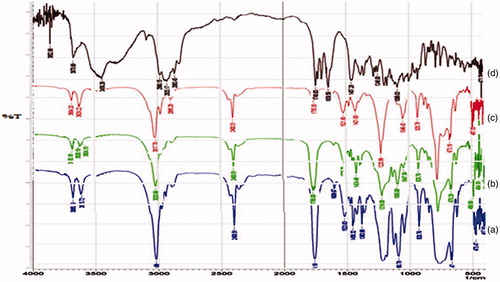
Figure 3. DSC thermograms of (a) Physical mixture of tacrolimus and PLA, (b) Tacrolimus and (c) PLA.
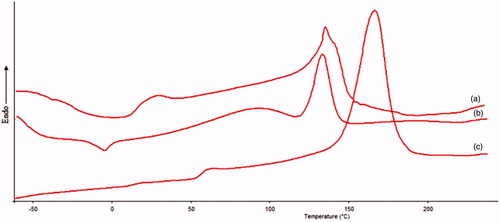
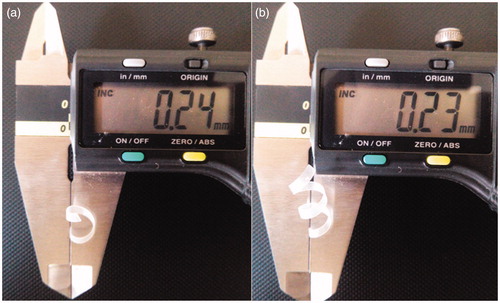
Figure 7. (a) Original diameter of stent, (b) Before keeping at freezing temperature, (c) After removal from freezing temperature. In-vitro behavior of stent after (d) 1 min, (e) 3 min and (f) 5 min.

Figure 8. (a) Original diameter of polymeric stent after insertion into Goat vessel, Ex-vivo behavior of stent (b) 1 min, (c) 3 min, (d) 5 min.

Figure 9. Hemocompatibility study (a) control untreated RBCs, (b) polymeric stent treated with RBCs.
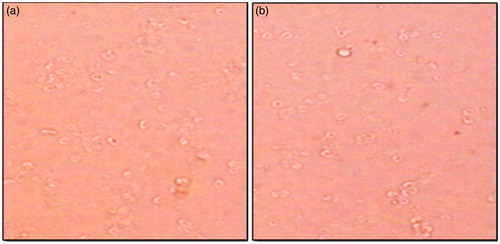
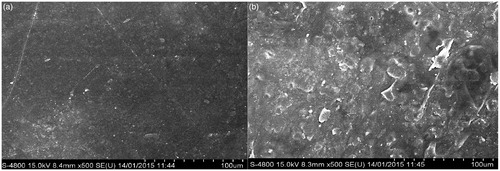
Figure 10. FESEM images of (a) Bare polymeric stent treated with platelets (b) Drug-loaded polymeric stent treated with platelets.
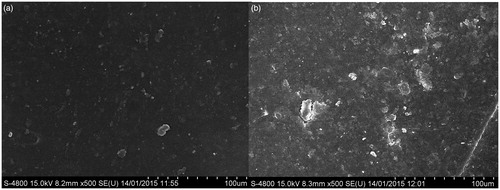
Figure 11. FESEM images of (a) Drug-loaded polymeric stent incubated in PBS solution (b) Drug-loaded polymeric stent incubated in distilled water (c) Drug-loaded polymeric stent (control).