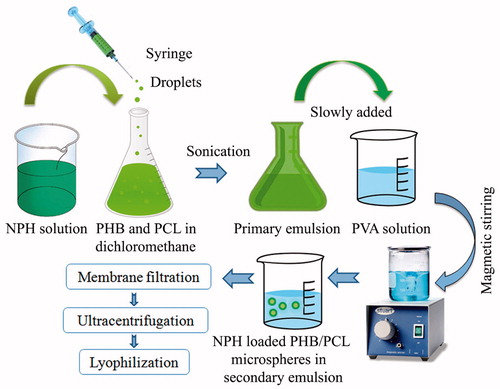
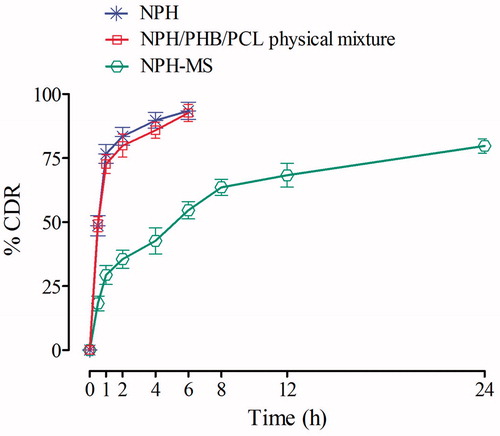
Figures & data
Figure 1. Chemical structure of (a) nefopam hydrochloride, (b) poly-3-hydroxybutyrate, and (c) poly-ɛ-caprolactone.
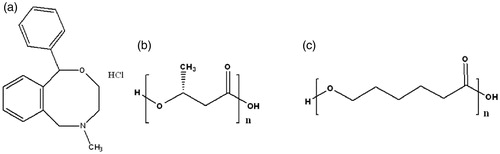
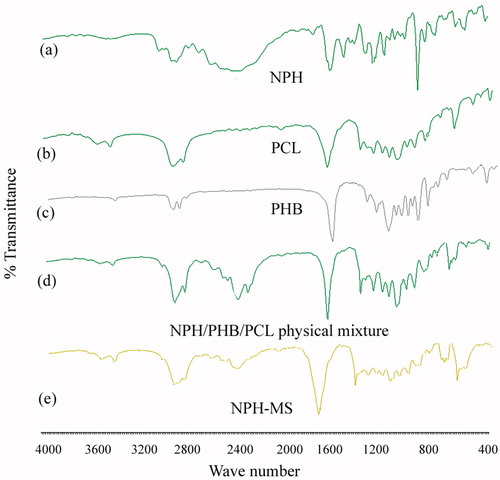
Figure 4. X-ray diffraction patterns of (a) NPH, (b) PCL, (c) PHB, (d) physical mixture, and (e) NPH-MS.
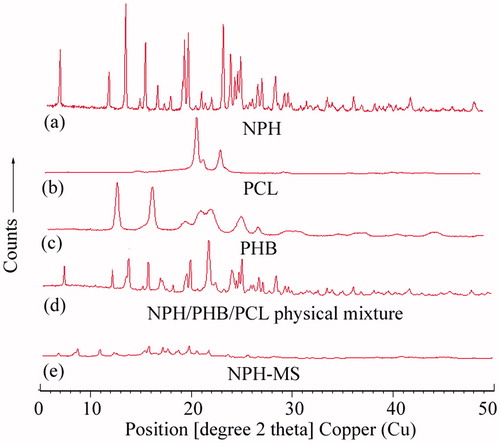
Figure 8. Tail withdrawal latency time from various doses of NPH-MS administered via peroral route (n = 5 rats per group). *p < .05, ***p < .001 compared with NPH treated rats.
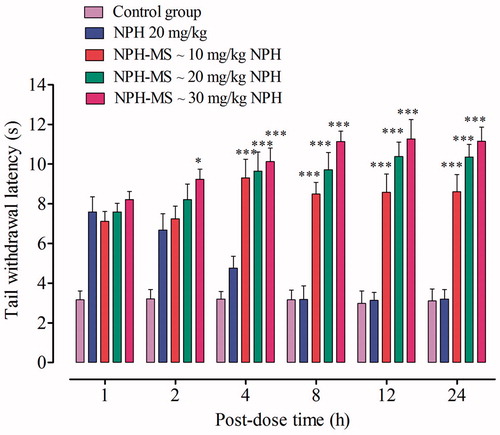
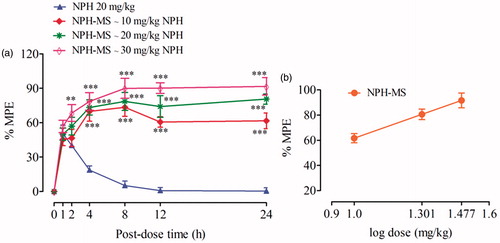
Figure 9. Anti-nociceptive effects of NPH-MS (p.o) in tail flick test (a) time-related effects, and (b) dose-related effects. **p < .01, ***p < .001 compared with NPH treated rats.