Figures & data
Table 1. Characterization of E1CLK-based niosomal formulation (composition, particle size, PD, EE% and zeta potential).
Figure 5. In-vitro release study of drug-loaded E1CLK niosomal vesicles at pH 7.4, 4.0 and 2.0 (n = 3, mean ± SEM).
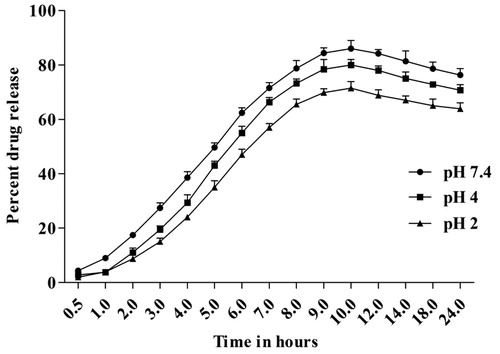
Figure 6. In-vitro haemolysis study of synthesized amphiphilic carrier (E1CLK) at different concentrations (n = 3, mean ± SEM).
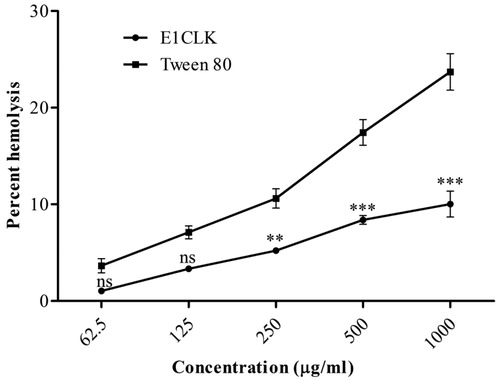
Figure 7. Cell cytotoxicity study of synthesized surfactant (E1CLK), where (A) and (B) show percent cell viability against NIH/3T3 cell line after 24 and 48 h, respectively, while (C) and (D) show percent cell viability against HeLa cell line after 24 and 48 h, respectively. (n = 3, mean ± SEM). Poly-L-lysine and Tween 80 are used as reference standard and positive control, respectively.
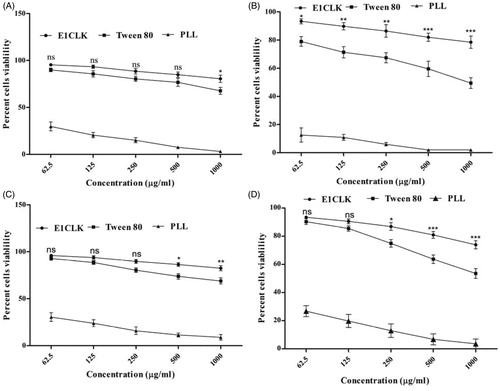
Table 2. In-vivo acute toxicity of E1CLK in mice.
Figure 8. Plasma drug concentration of clarithromycin when given orally as E1CLK niosomal formulation, commercial suspension and tablets at 15 mg/kg body weight dose (n = 6, mean ± SE).
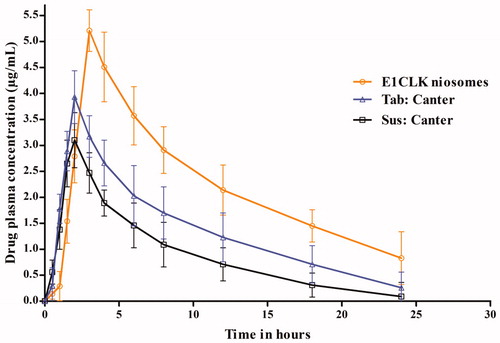
Table 3. In-vivo oral pharmacokinetics of clarithromycin when administered as E1CLK-based niosomal formulation, commercial suspension and tablets.