Figures & data
Figure 1. (a) Drug delivery nanosomes can be actively targeted to specific cells by ligand labelling such as folic acid, transcobalamin, iron transport proteins, hormones and growth factors to facilitate their active cellular import. Furthermore, the cargoes can be localized in subcellular location using specific organelle localization signal. Tumour cells can be passively targeted to using effect retention and permeability effect. (b) First-level drug delivery is the targeting of nanosomes to the capillary bed of a predetermined organ or tissue (green colour). Second-level targeting is the selective delivery of drugs to specific cell types such as tumour selective drug delivery to Kupffer cells in the liver (blue colour). Third-level targeting is drug delivery specifically to an intracellular site such as organelle targeting (red colour). Fourth level is drug delivery specifically to an intra-organelles compartment (yellow colour).
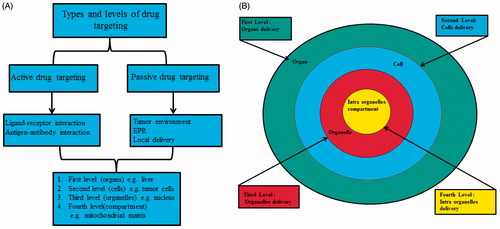
Table 1. The common nanosomes (NS) used as drug delivery cargoes with common cargo.
Table 2. Checkpoints that face nanosomes (NS) during their journey from first level to final destination.
Figure 2. Road map of nanosomes import and export across biological membranes. Abbreviations: CME: Clathrin-mediated endocytosis; CvME: Caveolae-mediated endocytosis; CCIE: clathrin- and caveolae-independent endocytosis; UPME: ubiquitin mediated endocytosis.
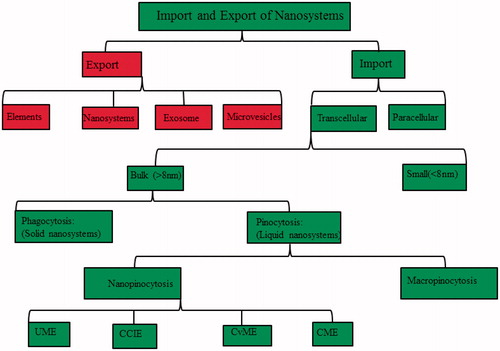
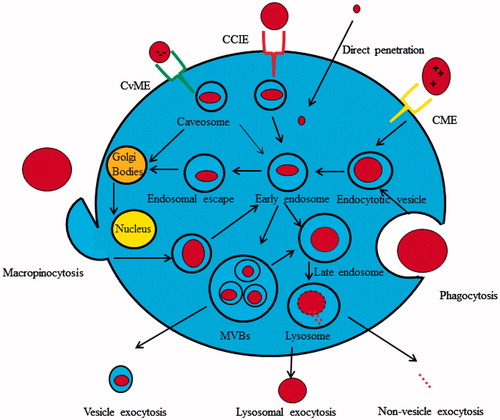
Figure 3. Endocytotic and exocytotic machinery of nanosomes, both of CME and CvME internalize cargoes across biological membranes. In particular those labeled with folic acid, transcobalamin, transferrin, hormones and growth factors. CME is the predominant path of internalization for positively charged nanosystems. However, CvME is recommended as portal for entry for anionic nanosystems. Thus, nano-drug delivery cargoes can be actively targeted to specific cells. The CCIE pathway of nanosystem import does not depend on the presence of clathrin or caveolin in the import of nanosystems. After internalization, the vesicles containing nanosystems are recycled to the plasma membrane, or targeted to late endosomes and later to lysosomes. The cargoes labelled to organelles targeting must escape the lysosomal degradation. Finally, nanosystems are exocytosed as elements, or as natural nanocarriers in the form of exosomes, microvesicles, or apoptosome.