Figures & data
Figure 1. Schematic representation of NPs and the progress of preparation. Dox and SPIONs were encapsulated in PLGA matrix via a double emulsion solvent evaporation method (W1/O/W2).
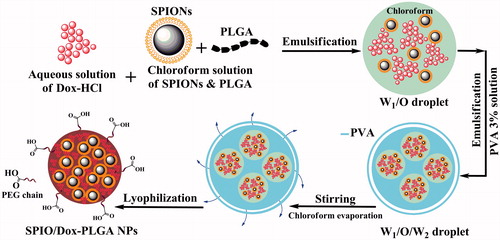
Figure 2. Schematic representation of NPs and the progress of preparation. Dox and SPIONs were encapsulated in PLGA matrix via a single emulsion solvent evaporation method (O/W).
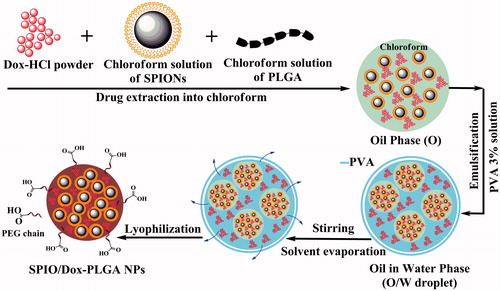
Figure 3. Schematic representation of NPs and the progress of preparation. Dox and SPIONs were encapsulated in PLGA matrix via a modified multiple emulsion solvent evaporation method (O1/W1/O2/W2).
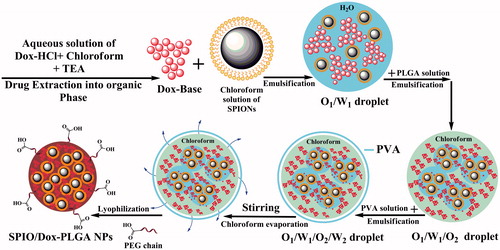
Figure 4. Schematic representation of NPs and the progress of preparation. Dox and SPIONs were encapsulated in PLGA matrix via a modified multiple emulsion solvent evaporation method (W1/O1,2/W2).
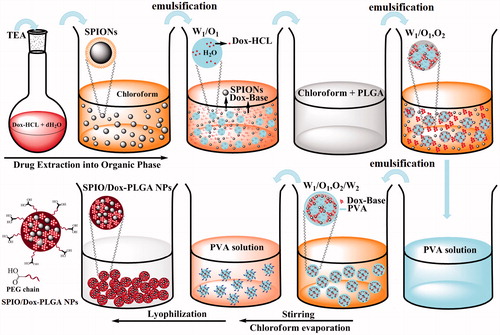
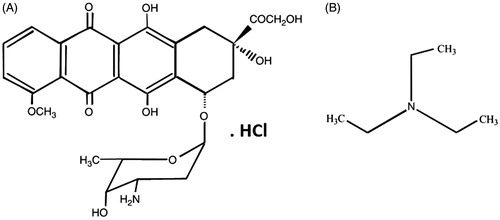
Figure 6. Aqueous solutions of Dox-HCl present a red colour at pH < pKa: 8.3 (A). A chemical conservation strategy was utilized to extract Dox-HCl into its free base form (protonated Dox which is hydrophobic) through a chemical reaction with TEA to improve Dox loading into the PLGA nanospheres (B). Whereas aqueous solution of Dox-HCl at pH above 10.5 shows a purple colour (C), Dox in the free base form shows an orange colour (D). Dox-loaded PLGA nanospheres (E) and SPIO/Dox loaded PLGA nanospheres (NPs) (F) with an excellent colloidal stability obtained by W1/O1,2/W2 methods.
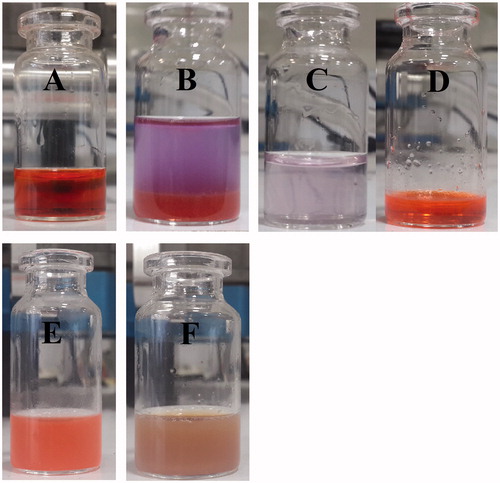
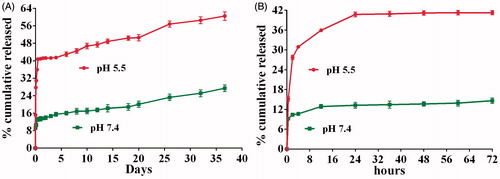
Figure 7. Particle size distribution of NPs prepared by W1/O1,2/W2 method. (A, B) The particle size distribution of NPs using DLS and AFM analysis, respectively.
Table 1. Ingredients for preparation of NPs as well as particle size, PDI, LC and EE.