Figures & data
Figure 1. The allene oxide synthase pathway resulting in the production of cis(+)-12-oxophytodienoic acid from α-linolenic acid (18:3). The pathway requires the cooperative action of 13-LOX, AOS and AOC. In the absence of AOC, the unstable 12,13-EOT intermediate is non-enzymatically converted to α-ketol, γ-ketol and the racemic mixture of 12-OPDA as indicated by the dotted pathway.
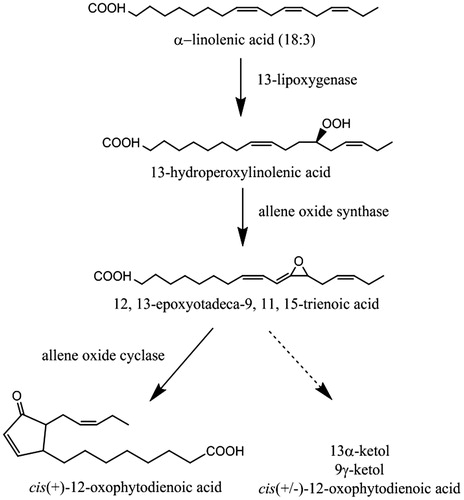
Table 1. Immobilization efficiency of the covalent immobilization of proteins on modified RHS.
Table 2. Comparison of the specific activities of soyLOX and OsAOS1 in free and covalently immobilized systems on GDA- and ECH-PEG-linked RHS.
Figure 2. The concentration-dependent comparison of reaction kinetics of free and immobilized soyLOX. LnA (0.1 mM) was used as the substrate. HPOTE was continuously measured in the free soyLOX system (A) and discontinuously measured in the immobilized soyLOX systems on GDA- (B) and ECH-PEG-linked RHS (C). Data from the discontinuous enzyme assays are represented as the mean ± SD (n = 3).
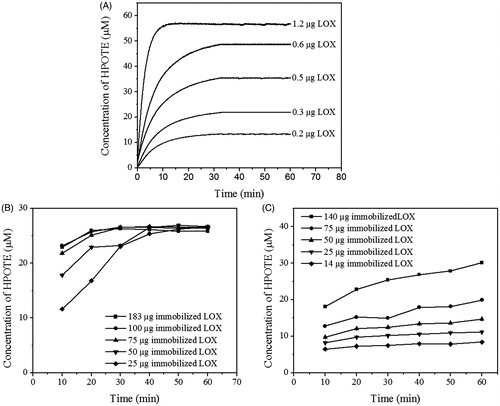
Figure 3. The production of cis-OPDA as a function of the molar ratio of OsAOC to OsAOS1 in the co-immobilized system of OsAOS1 and OsAOC on GDA-linked RHS. HPOTE (0.5 mM) was used as the substrate for the co-immobilized AOS-AOC on GDA-linked RHS. The reaction was carried out in 0.05 mM sodium phosphate buffer (pH 8.0) at RT for 1 h. Data are represented as the mean ± SD (n = 3).
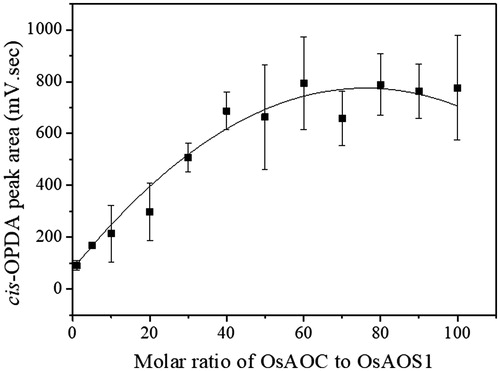
Table 3. Relative product yields of immobilized enzymes on modified RHS.
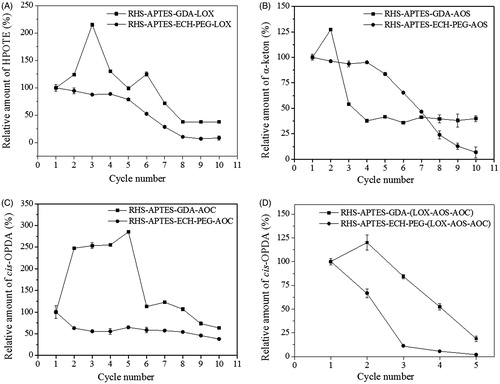
Figure 4. Reusability of immobilized soyLOX, OsAOS1 and OsAOC on GDA- and ECH-PEG-linked RHS. (A) Immobilized soyLOX. (B) Immobilized OsAOS1. (C) Immobilized OsAOC. (D) Co-immobilized LOX-AOS-AOC. Product amounts were calculated relative to the amount of product generated in the first cycle of enzyme use. Data are represented as the mean ± SD (n = 3).