Figures & data
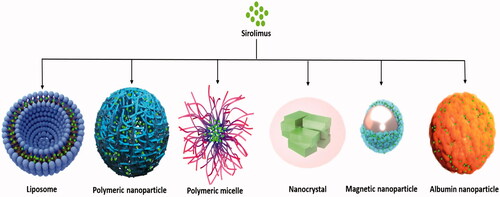
Table 1. Nanocarrier compositions and characteristics of sirolimus liposomal formulations.
Table 2. Nanocarrier compositions and characteristics of sirolimus polymeric nanoparticles.
Table 3. Nanocarrier compositions and characteristics of sirolimus micelles.
Table 4. Nanocarrier compositions and characteristics of sirolimus incorporated in miscellaneous nanoformulations.

![Figure 2. Indications for which sirolimus has been investigated. A growing number of evidence has demonstrated that sirolimus is beneficial for treating various diseases [Citation13–27]. GVHD: Graft-versus-host disease; HIV: Human immunodeficiency virus; LAM: Lymphangioleiomyomatosis.](/cms/asset/d916dfa1-1865-4e08-b74e-1749b647a33b/ianb_a_1408123_f0002_c.jpg)
![Figure 4. Chitosan decorated sirolimus liposomes attenuated vascular restenosis following local delivery as confirmed by pathology, immunohistochemical and in vivo CT angiography. Reprinted from ref [Citation46]. Copyright (2017), with permission from Elsevier.](/cms/asset/095bae75-156a-4507-a0db-dc706061ab74/ianb_a_1408123_f0004_c.jpg)
![Figure 5. Schematic structure of the theranostic targeted thermosensitive liposome (A) and its antitumour mechanism (B). Redrawn with permission from Pang et al. [Citation47]. Copyright (2016) American Chemical Society.](/cms/asset/78bc6671-5a06-4312-91f2-86e91a81167a/ianb_a_1408123_f0005_c.jpg)
![Figure 6. Design of sustained sirolimus delivery nanomedicines based on acetalated β-CD (A), sirolimus concentrations in the blood and the aorta of C57BL/6 mice (n = 4, mean ± SEM), after subcutaneous administration of sirolimus nanoparticles at 3 mg/kg of drug (B). Therapy with sirolimus nanoparticle significantly reduced atherosclerosis in ApoE−/−mice (C). (C) Representative photographs of total aortas from each group and quantitative results subjected to various treatments. Reprinted with minor modification from Dou et al. [Citation97]. Copyright (2016), with permission from Elsevier.](/cms/asset/49011059-bda2-45d8-9004-24722ea67f1a/ianb_a_1408123_f0006_c.jpg)
