Figures & data
Table 1. The geometrical parameters (bond distance in Å) and topological parameters (in a.u.) of in more stable conformers.
Table 2. Topological parameters (in a.u.) and intramolecular hydrogen bond energies (in kJ/mol) of stable and unstable conformers of benserazide.
Table 3. The NBO analysis of stable and unstable conformers of benserazide (includes the second order perturbation energy of some important orbital interactions).
Table 4. Total energy, dipole moment, and MO analyses of stable and unstable conformers of benserazide.
Table 5. The harmonic stretching vibrational frequencies of O…H and N…H for the most stable conformations of benserazide.
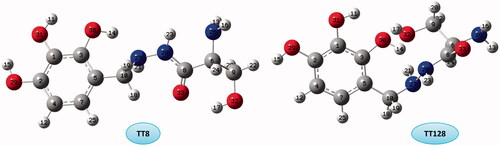
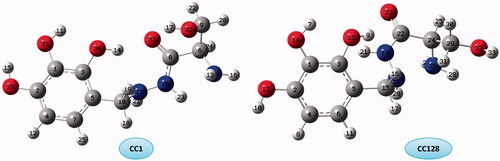
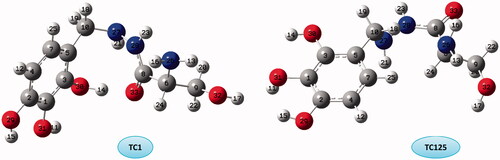
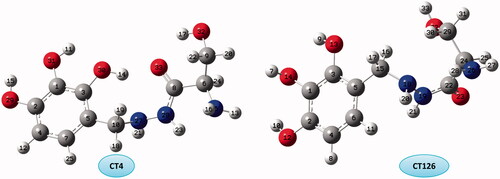
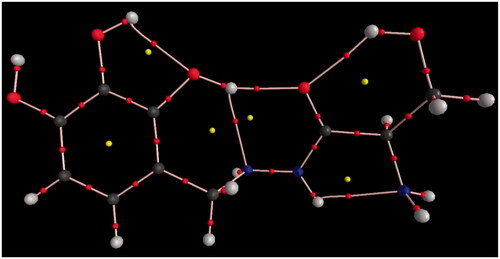
Figure 8. Molecular graphs of the conformers calculated at B3LYP/6–311 G(d) level using AIM approach.
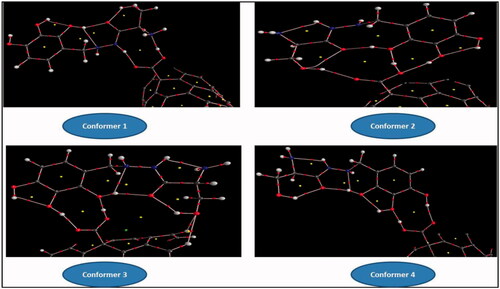
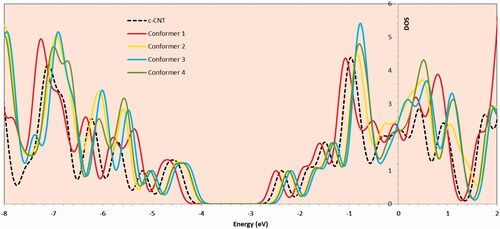
Table 6. Adsorption energy, Eads in kJ/mol, optimum distance of interaction, in Ǻ, electron density (ρBCP), Laplacian (∇2ρBCP), electron kinetic energy density (GBCP), electron potential energy density (VBCP), total electron energy density (HBCP) and hydrogen bond energy (EHB) at the bond critical point (BCP) of all configurations calculated at B3LYP/6–311 G(d) level.
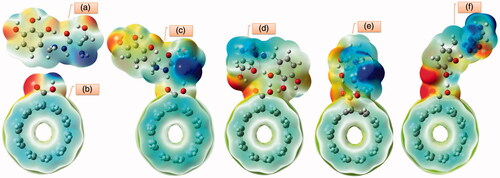
Figure 9. Frontier molecular orbitals (HOMO-LUMO) plots with corresponding energy gap of (a) carboxylated carbon nanotube and (b) benserazide molecule.
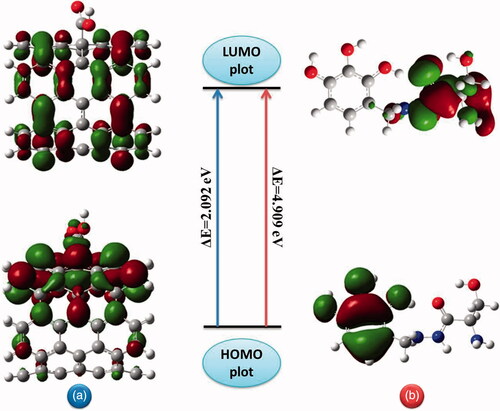
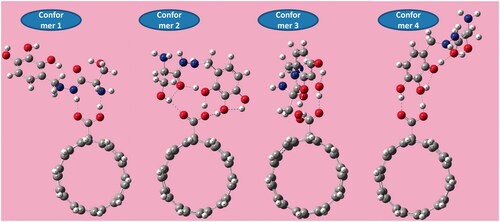
Figure 10. The pictorial illustration of HOMO and LUMO frontier molecular orbitals with corresponding energy gap for different possible complexes calculated by B3LYP method with 6–311 G(d) basis set; (a) conformer 1, (b) conformer 2, (c) conformer 3 and (d) conformer 4.
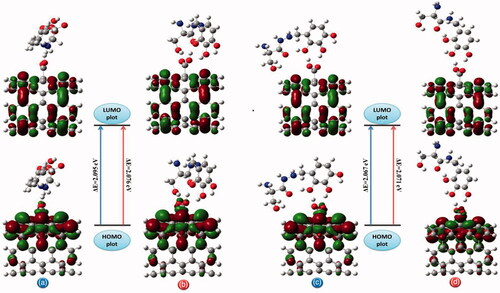
Table 7. Total energy (Hartree) and global reactivity descriptors (all units in eV) for BZ/c-CNT drug carrier conformers and its constituents.