Figures & data
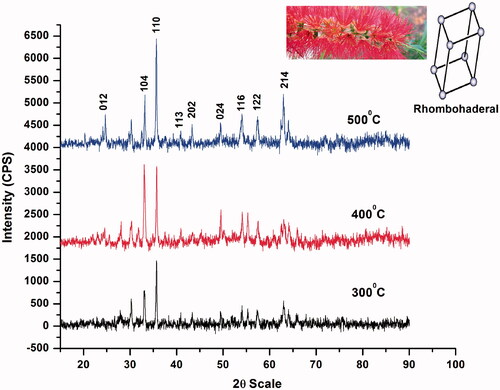
Table 1. Size and lattice strain measured using XRD.
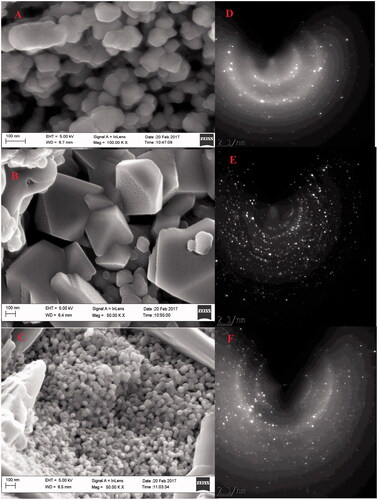
Figure 5. (A–C) HR-TEM images of biogenic IONPs annealed at 300 °C; (D–F) at 400 °C; (G–I) at 500 °C.
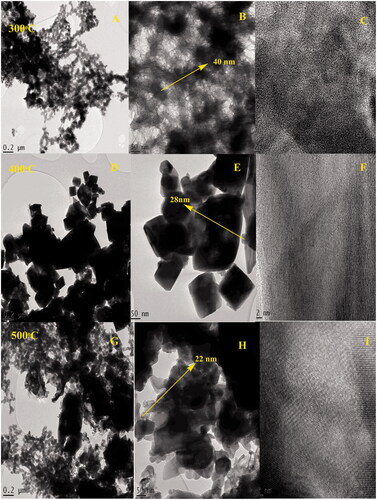
Figure 7. (A) FTIR spectrum of biogenic IONPs at 300, 400 and 500 °C (B) Magnetization curves of biogenic iron oxide nanoparticles.
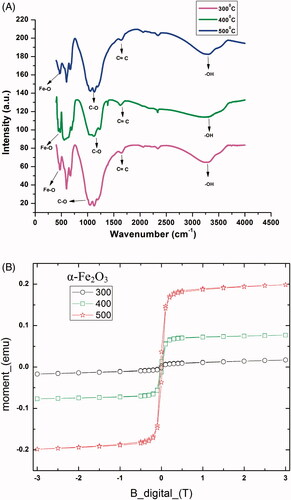
Table 2. Coercivity values measured for each IONP sample.
Figure 10. Possible cytotoxic mechanism. (A) Extracellular ROS generations (which can easily penetrateinside the cell) followed by their interference with nuclear material, leading to genotoxicity. (B) IONPs are internalized by receptor-mediated endocytosis, followed by their entry into the lysosomes, where Fe2+ dissolution occurs. (C) Surface defects in IONP?s can result in rupturing of cellular membranes. The mechanism have been developed through the work ofearlier researchers. (D) The generated ions not only interfere with other proteins, but also make their way into the mitochondria, where they disrupt its function by creating further ROS. Adapted from [Citation11].
![Figure 10. Possible cytotoxic mechanism. (A) Extracellular ROS generations (which can easily penetrateinside the cell) followed by their interference with nuclear material, leading to genotoxicity. (B) IONPs are internalized by receptor-mediated endocytosis, followed by their entry into the lysosomes, where Fe2+ dissolution occurs. (C) Surface defects in IONP?s can result in rupturing of cellular membranes. The mechanism have been developed through the work ofearlier researchers. (D) The generated ions not only interfere with other proteins, but also make their way into the mitochondria, where they disrupt its function by creating further ROS. Adapted from [Citation11].](/cms/asset/19ec74ad-d4fd-479b-b1ff-f70f0b92e428/ianb_a_1434534_f0010_c.jpg)
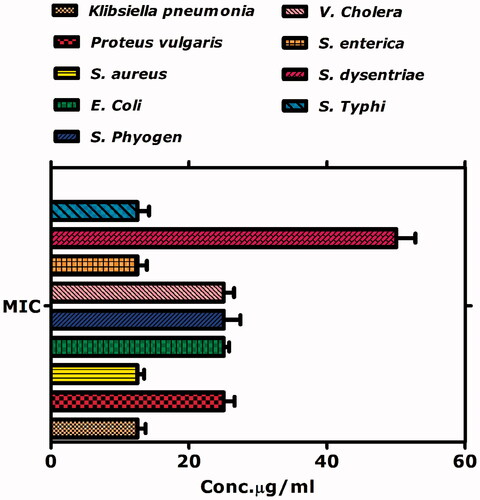
Table 3. Antibacterial activities of biogenic iron oxide nanoparticles.
Figure 13. Schematic representation of the inhibition of choline esterase by magnetic IONPs. (A,B) indicate the formation of acetyle choline which is catalyzed by acetyle choline transferase enzyme in the transmitting neurons. (C) indicate the normal conditions where acetyle choline can reach the reciveing neurons. (D) in the presence of choline esterases, acetyle choline is degraded and is unable to transmit the signal. (E) shows nanoparticles inhibitng the choline esterase enzyme which allows the neurotransmitter to transmit the signal.
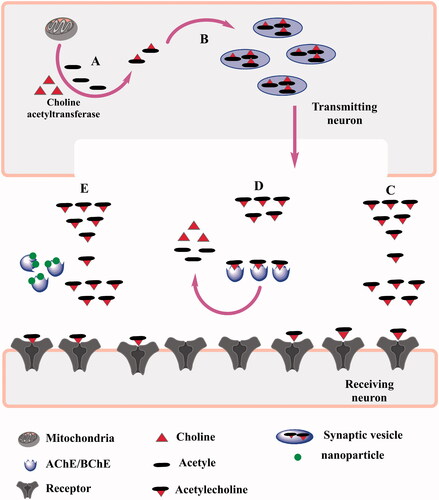
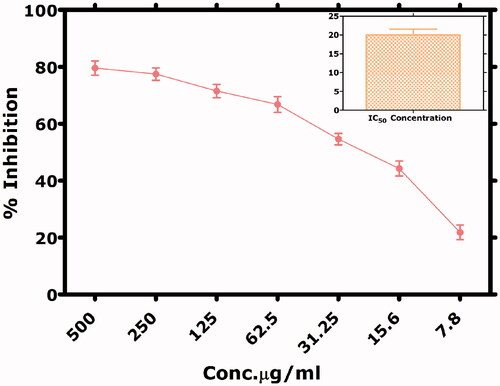
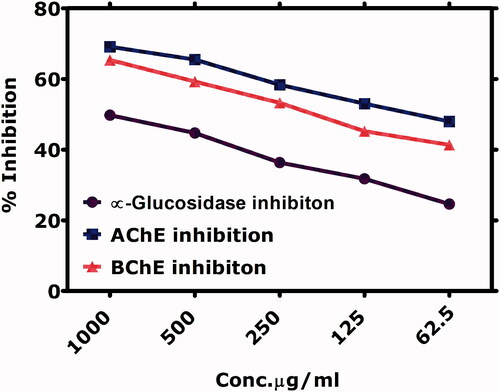
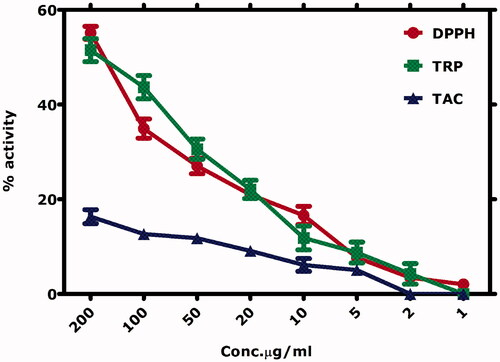
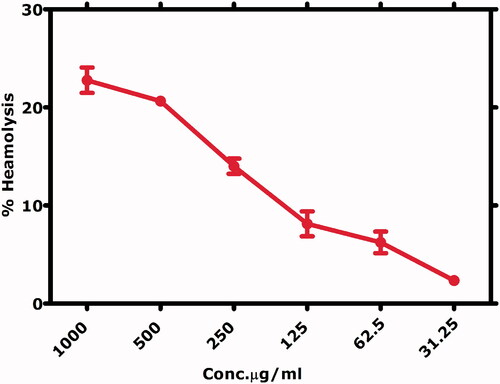
Table 4. IC50 Values calculated for various biological assays.