Figures & data
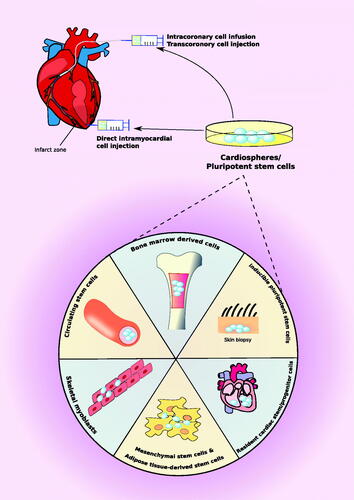
Table 1. Application of stem cell therapy for the treatment of cardiovascular diseases.
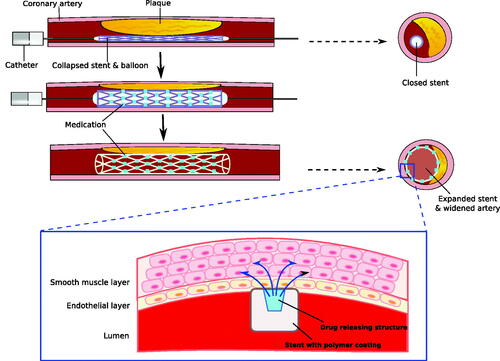
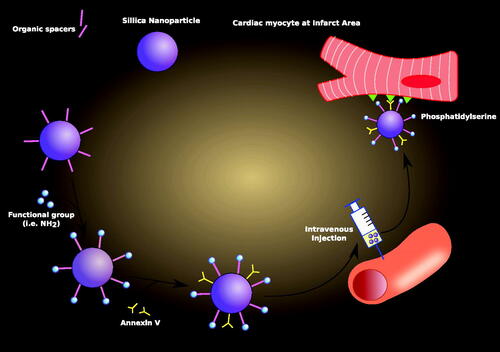
Table 2. Targeted drug delivery for cardiovascular diseases using nanoparticles.