Figures & data
Figure 1. Fabrication of the oesophageal scaffold reinforced by 3D-printed rings. The scaffold blended with reinforcement rings was fabricated by combining 3D printing and electrospinning methods. (A) The first step involved the fabrication of PCL rings on a stainless rod. (B and C) After creating the serial PCL rings, tubular PCL was collected from electrospinning. (D) The final oesophageal scaffold with reinforced rings. Abbreviations: PCL: Poly ε-caprolactone.
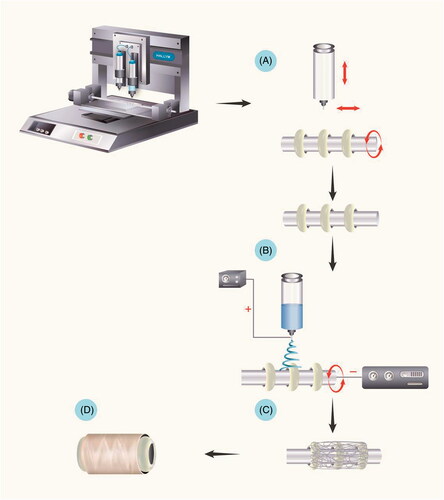
Figure 2. Serial process of scaffold culture and implantation. (A) Oesophageal scaffold implantation into the greater omentum. (B) The scaffold was cultured in the greater omentum for two weeks. (C) Orthotopic oesophageal scaffold implantation into the cervical oesophagus.
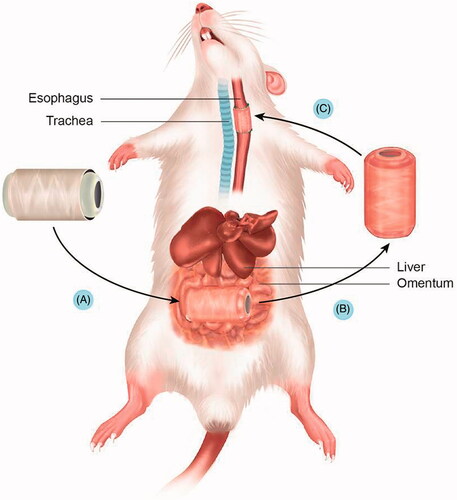
Figure 3. Gross morphology of oesophageal implantation into the greater omentum. (A) The greater omentum was brought out of the peritoneal cavity. (B) The oesophageal scaffold was embedded into the lower portion of the greater omentum and wrapped using 4–0 Vicryl sutures. (C) After two weeks of implantation, the cultured scaffold was harvested. (D) Orthotopic-cultured oesophageal scaffold implantation into the cervical oesophagus.
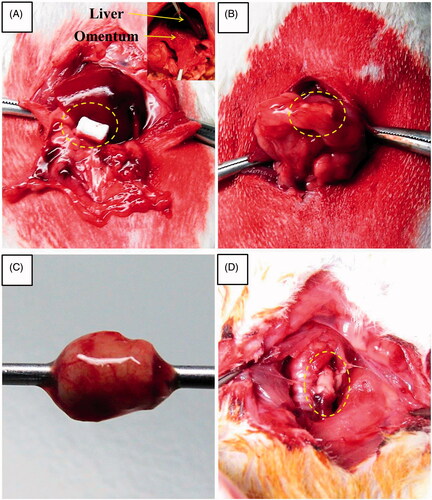
Figure 4. Scanning electron microscopy images of the PCL scaffold with reinforcement ring. (A and B) The scaffold was composed of a PCL with reinforcement rings. (C) The nano-fibre-based structure of the scaffold. (D) The highly porous structure of the scaffold. (E and F) Gross appearance of hybrid scaffolds reinforced by a 3D-printed ring before implantation. (G and H) The diameter-distribution histogram. PCL: Poly ε-caprolactone.
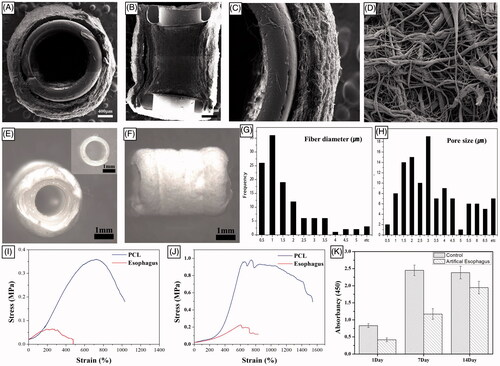
Figure 5. Macroscopic appearance of reconstructed artificial neo-oesophagus constructs (A and B). After two weeks of greater omentum culture, the embedded implant was retrieved from the omentum. The cultured oesophageal graft was intact, and tissue regeneration from the greater omentum inside and outside of the scaffold was observed. The regenerated scaffold surface alignment became symmetric. Gross morphology after the implantation. (C) Orthotopic-cultured oesophageal scaffold implantation into the cervical oesophagus. (D) Gross morphology of reconstructed artificial oesophagus constructs after the second week from the implant. There was no evidence of a fistula, perforation, abscess formation or surrounding soft-tissue necrosis. The dotted line shows the continuity of the artificial oesophagus and the native oesophagus.
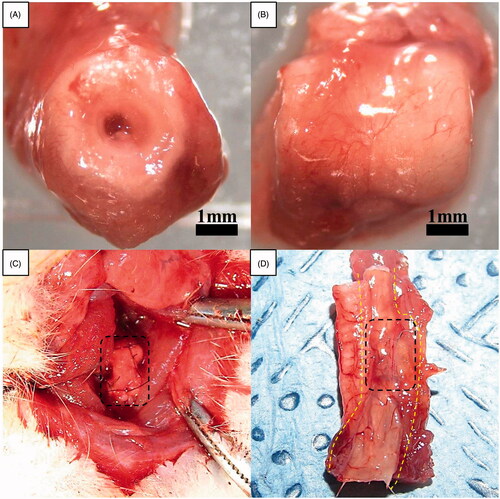
Figure 6. Histological results for the haematoxylin-eosin–stained axial (A) and longitudinal (B) section of omentum-cultured scaffold. The scaffold material was integrated into the omentum, and a mixture of inflammatory cells and fibroblasts infiltrated inside and outside of the scaffold. The host cells were aligned with the cylindrical direction inside (C) and outside (D) of the scaffold. (E) Several newly developed blood vessels in the outer side of the scaffold revealed the tissue regeneration process. Immunohistochemistry of omentum-cultured scaffold. DAPI (4,6-diamidino-2-phenylindole) staining (F). Rhodamine phalloidin staining for F-actin (G). The merged file of DAPI (4,6-diamidino-2-phenylindole)/F-Actin (H) indicated that the host cells infiltrated into the scaffold and along the inner/outer wall of the scaffold.
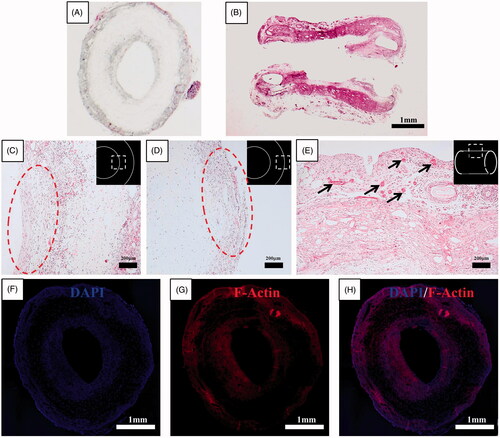
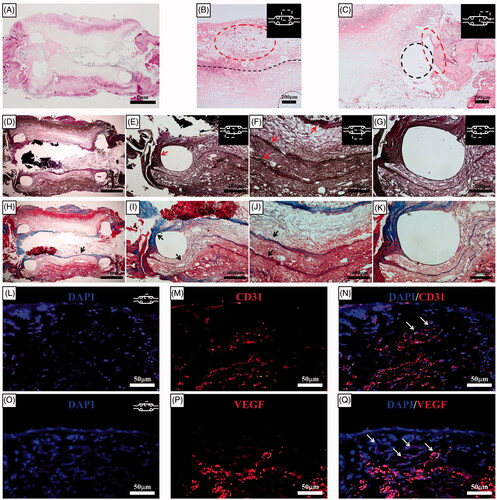
Figure 7. Histological results for the haematoxylin-eosin–stained sections two weeks after orthotopic scaffold implantation. (A) At the end of the second week, a PCL with reinforcement rings could be identified. The artificial oesophagus constructs showed healing of the circumferential defects by the second week. The fusion of the PCL scaffold and regenerative tissue was intact. Mild inflammatory reactions and minimal granulation tissue formation were observed. (B) Several newly developed blood vessels in the outer side of the scaffold indicated tissue regeneration. The structural integrity was maintained by the alignment of infiltrated host cells into the scaffold. The dotted line represents the boundary between the omentum-cultured scaffold and the regenerated tissue covering the scaffold. (C) Squamous epithelium and soft-tissue regeneration occurred at the periphery of the oesophageal defect. Histological analysis for the Verhoeff's van Gieson Stain (D, E, F, G) and Masson's trichrome (H, I, J, K) sections two weeks after implantation. Elastin (arrows in E and F) and collagen (arrows in H, I, and J) fibre deposition were observed in all parts of the artificial oesophagus with layered structure. The tissue regeneration originated from the natural oesophagus and connected to either end of the artificial oesophagus. The dotted line represents the PCL ring. Immunohistochemistry of the implanted scaffold. DAPI (4,6-diamidino-2-phenylindole) staining (L and O). Platelet endothelial cell adhesion molecule (CD31/PECAM-1) (M) and VEGF staining (P). The merged file of DAPI/CD31 (N) and DAPI/VEGF (Q) indicated that neovascularization developed in the implanted scaffold (white arrows). PCL: Poly ε-caprolactone.