Figures & data
Figure 1. The rats are divided into 3 groups each receives 4 weekly infusion of one of the following samples: lactated ringer’s solution (LR) as control group, bovine polySFHb (Phe) and bovine poly-[Hb-CAT-SOD-CA] (PHE) with enhanced enzyme activity. This is followed on week 5 by a 30% exchange infusion.
![Figure 1. The rats are divided into 3 groups each receives 4 weekly infusion of one of the following samples: lactated ringer’s solution (LR) as control group, bovine polySFHb (Phe) and bovine poly-[Hb-CAT-SOD-CA] (PHE) with enhanced enzyme activity. This is followed on week 5 by a 30% exchange infusion.](/cms/asset/631b072a-5695-41e7-b892-3f5ef92fb8cf/ianb_a_1476375_f0001_c.jpg)
Figure 2. Body weight changes of the rats during the four-week Top-loading infusion period. The body weight is measured in gram (g). Each figure shows a group of rats injected with corresponding sample (control LR, PHe, PHE). The body weight in rats injected with control (LR) are 158 ± 3.7 g, 227 ± 7 g, 288 ± 17 g, 348 ± 20 g. The body weight in rats injected with bovine PHe are 183 ± 9 g, 248 ± 15 g, 313 ± 9 g, 366 ± 25 g. The body weight in rats injected with bovine PHE are 198 ± 4 g, 257 ± 5 g, 318 ± 9 g, 367 ± 9 g. There are no significant differences from the control group.
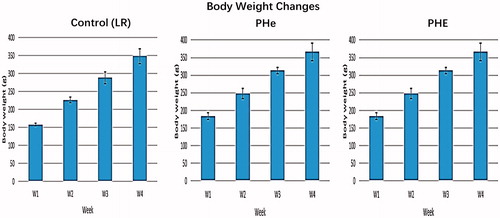
Figure 3. BUN urea, creatinine and CK levels. All data are in . No significant statistical differences from the control groups.
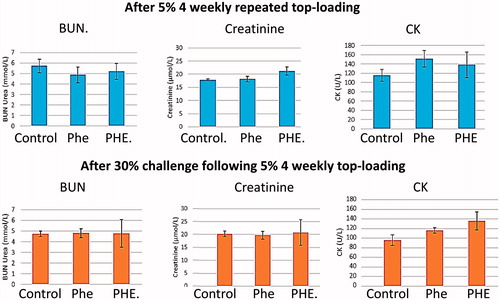
Figure 4. Albumin, total bilirubin, ALT and AST levels. All data are in . No significant statistical differences from the control groups.
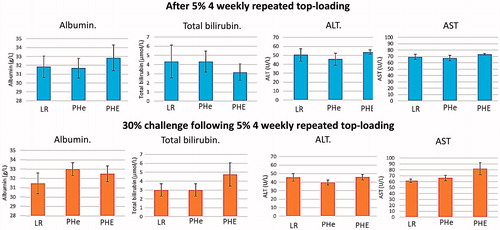
Table 1. Biochemistry test.
Figure 5. Ouchterlony double diffusion test. Positive control of BSA showing BSA and anti-BSA antibody precipitation.
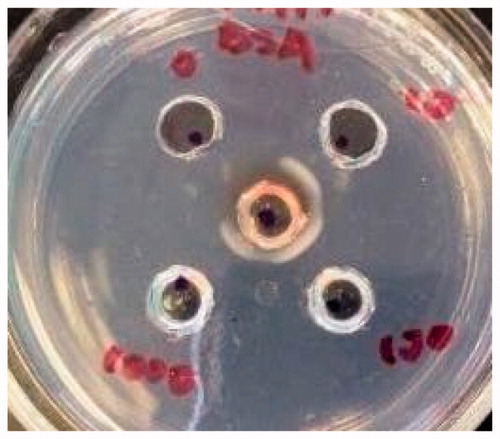
Figure 6. Ouchterlony double diffusion test on rats after 4 weekly 5% top-loading infusion with lactated ringer’s solution as control, Bovine PHe or PHE (6 rats/group) showing no antigen-antibody precipitations.
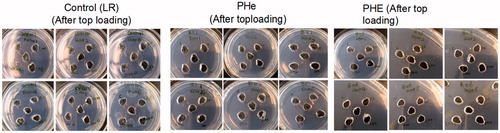
Figure 7. Ouchterlony double diffusion test on rats after 30% blood volume exchange transfusion with control (LR), PHe, or PHE (4 rats/group)) showing no antigen-antibody precipitations.

Figure 8. Total IgG levels after four-weekly 5% blood volume top-loading infusions followed by 30% blood volume exchange transfusion. After four-weekly injection-period, the total IgG levels in rat plasma against LR control group, PHe and PHE are 5.42 ± 2.36 mg/ml, 7.88 ± 3.13 mg/ml and 10.41 ± 7.80 mg/ml, respectively; p values by one-way ANOVA is .32 (>.05). After 30% blood volume exchange transfusion, the total IgG concentrations in LR control group, PHe and PHE group are 5.84 ± 1.86 mg/ml, 7.82 ± 3.27 mg/ml and 9.24 ± 5.80 mg/ml; p values by one-way ANOVA is .60 (>.05).
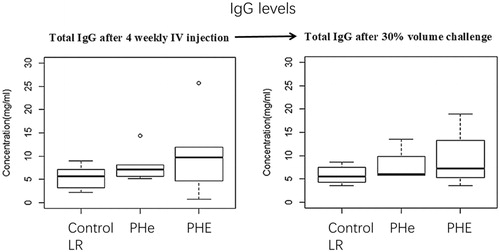
Figure 9. Total IgM levels after four-weekly 5% blood volume top-loading infusions followed by 30% blood volume exchange transfusion. Total IgM levels after top-loading infusion with LR control group, PHe or PHE are 0.56 ± 0.33 mg/ml, 1.24 ± 0.60 mg/ml and 2.28 ± 0.87 mg/ml; p values by one-way ANOVA is .002 (<.05). Total IgM levels after 30% blood volume exchange transfusion with LR control group, PHe or PHE are 0.57 ± 0.22 mg/ml, 1.79 ± 0.75 mg/ml and 2.11 ± 1.33 mg/ml; p values by one-way ANOVA is .14 (>.05). However, if we only compare the control group and PHe group by student’s T-test, the p value is .035 (<.05); compare the control group and PHE by student’s T-test, p value is .093 (>.05).
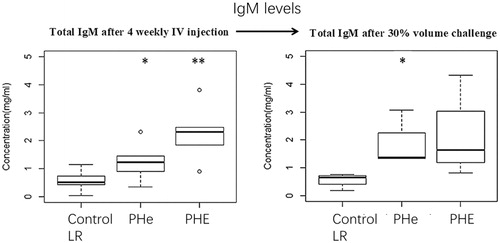
Figure 10. The excess hemoglobin in the poly-[Hb-CAT-SOD-CA] complex nanoencapsulate the more antigenic enzymes.
![Figure 10. The excess hemoglobin in the poly-[Hb-CAT-SOD-CA] complex nanoencapsulate the more antigenic enzymes.](/cms/asset/7e0b9d7c-41d0-4145-8169-120410ea83f2/ianb_a_1476375_f0010_c.jpg)
Figure 11. The antibodies levels against SOD after 4-weekly Top-loading infusions in group of control (LR), PHe and PHE are 1.92 ± 2.09 ng/ml, 1.30 ± 2.11 ng/ml and 1.28 ± 2.57 ng/ml respectively. p Values by one-way ANOVA is .48 (> .05). The antibodies against bovine SOD in group of control (LR), PHe and PHE are 21.13 ± 7.57 ng/ml, 28.79 ± 15.07 ng/ml and 6.80 ± 7.79 ng/ml; p values by one-way ANOVA is .09 (> .05).
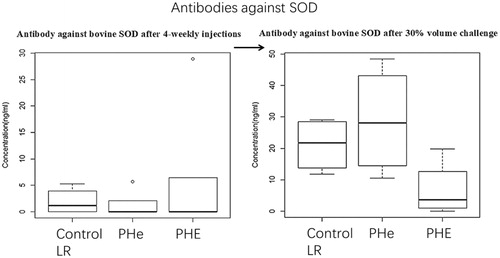
Figure 12. The concentration of antibodies against bovine Hb after four-weekly injection in group of control (LR), PHe and PHE are 0.41 ± 0.09 mg/ml, 0.49 ± 0.13 mg/ml and 0.42 ± 0.08 mg/ml, respectively. The p values by one-way ANOVA is .83 (> .05). After 30% blood volume exchange, the antibodies against Hb in group of control (LR), PHe and PHE are 0.44 ± 0.14 mg/ml, 0.40 ± 0.10 mg/ml and 0.52 ± 0.11 mg/ml, respectively; p values by one-way ANOVA is .47 (> .05).
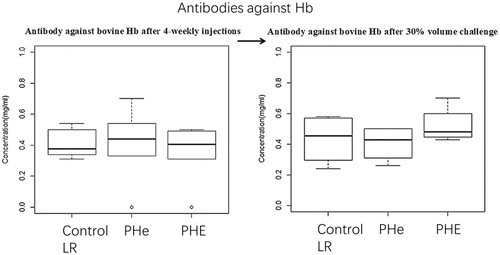
Figure 13. Antibody against bovine CAT after 4-weekly injections followed by 30% blood volume exchange transfusion. No antibody is detected.
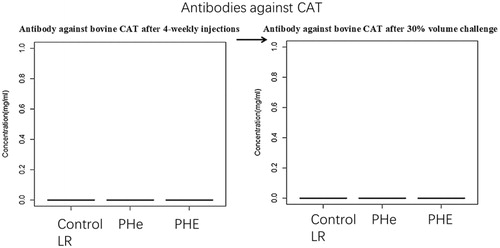
Figure 14. Antibody against bovine CA after four-weekly injections followed by 30% blood volume exchange transfusion. No antibody is detected.
Figure 15. C3a activation test. After four-weekly injections, C3a levels in LR control group, PHe and PHE groups are 144.49 ± 4.39 ng/ml, 150.74 ± 6.38 ng/ml and 153.56 ± 9.90 ng/ml, respectively; p values by one-way ANOVA is .16 (>.05). After four-week top-loading infusions followed by 30% blood volume exchange, C3a levels in LR control group, PHe and PHE groups are 150.74 ± 1.60 ng/ml, 149.70 ± 2.77 ng/ml and 150.50 ± 3.18 ng/ml, respectively; p values by one-way ANOVA is .88 (>.05).
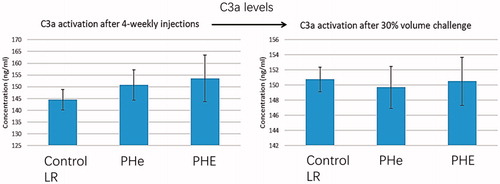
Figure 16. Mean arterial pressures are recorded just before and 5 min after each top-loading infusion. Each figure shows a group of rats injected with corresponding sample. Control (LR): the correlation coefficients between the blood pressure before or after injection are 0. 27 and 0.56, respectively. The p values of the coefficients are 0.73 and 0.44, respectively. PHe: the correlation coefficients between the blood pressure before or after injection are 0.89 and 0.77, respectively. The p values of the coefficients are 0.11 and 0.23, respectively. PHE: the correlation coefficients between the blood pressure before or after injection are 0.16 and 0.30, respectively. The p values of the coefficients are 0.24 and 0.25, respectively.
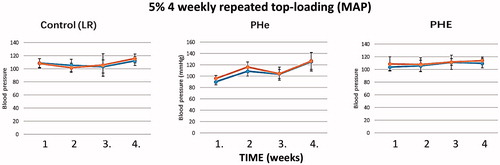
Figure 17. Mean arterial pressure during the 30% blood volume exchange transfusion. Each figure showed a group of rats infused with one of lactated ringer’s solution (control), PHe, PHE. Control (LR): the correlation coefficients of the blood pressure before and after infusion is 0.13. The p values of the coefficient is .55. PHe: the correlation coefficient between the blood pressure before or after infusion is −0.03. The p values of the coefficient is .87. PHE: the correlation coefficients between the blood pressure before or after infusion are −0.16. The p values of the coefficient is .45. Thus, there are no significant changes.
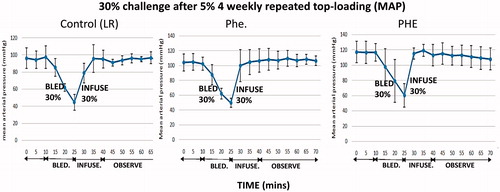
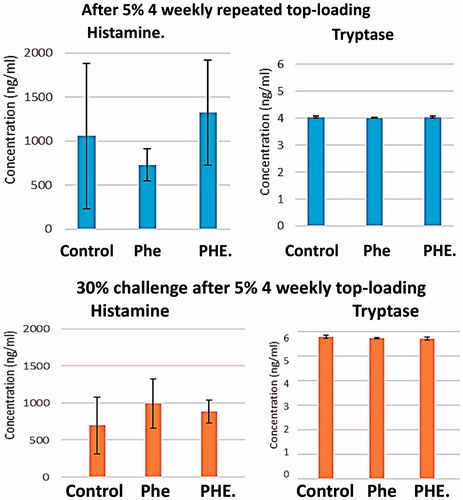
Figure 19. Histamine and tryptase levels tested before and 30 min after 30% blood volume exchange transfusion of LR (control), PHe or PHE. Plasma trptase analysis is more accurate than histamine as shown by the higher SD for histamine. The histamine levels: the p values by one-way ANOVA among the three groups is .62 (>.05). The tryptase levels: comparing the LR control groups and the PHe and PHE groups, the p values by one-way ANOVA is .51 (>.05) before infusion, and the p values of after infusion is .21 (>.05). Thus, there are no significant changes in the histamine or tryptase levels between the control group and the PHe and PHE groups.