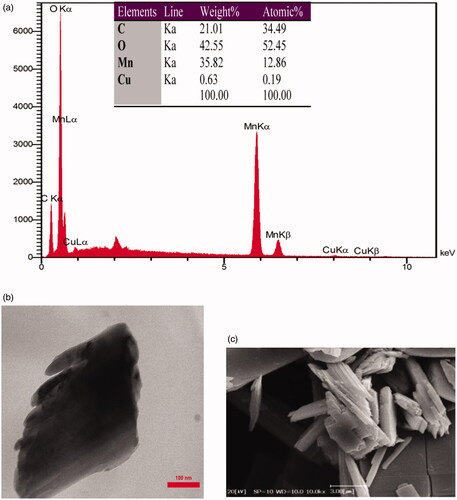
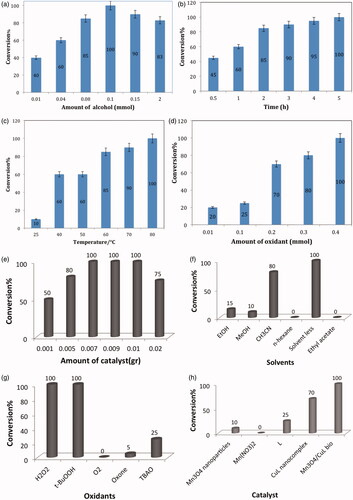
Figures & data
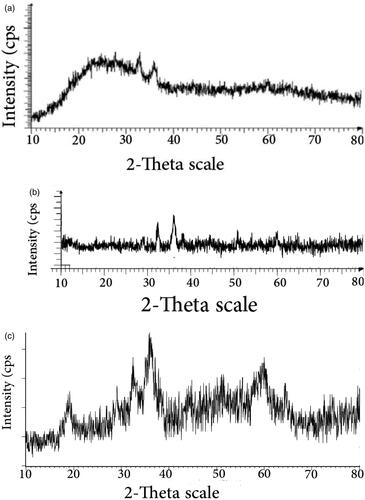
Figure 1. XRD of synthesized product from manganese salt in the presence of plant at pH = 7 (a), at pH = 9 (b) and XRD of Mn3O4/CuL bio-nanocolloid (c).
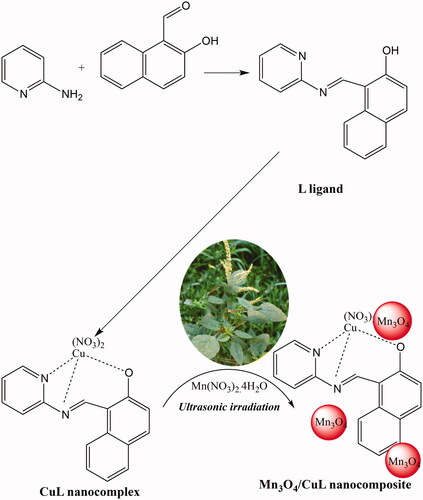
Scheme 2. The oxidation of primary and secondary alcohols catalysed by Mn3O4/CuL bio-nanocolloid using H2O2 to have O2 and water as byproducts under ultrasonic irradiation.
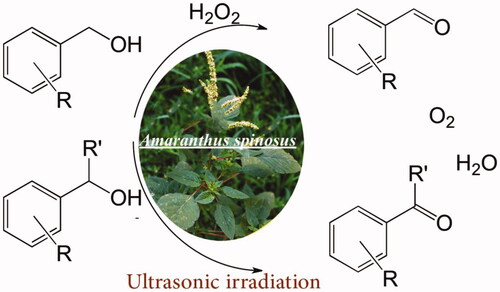
Figure 6. Proposed mechanism for the catalytic oxidation of benzyl alcohol by Mn3O4/CuL bio-nanocolloid.
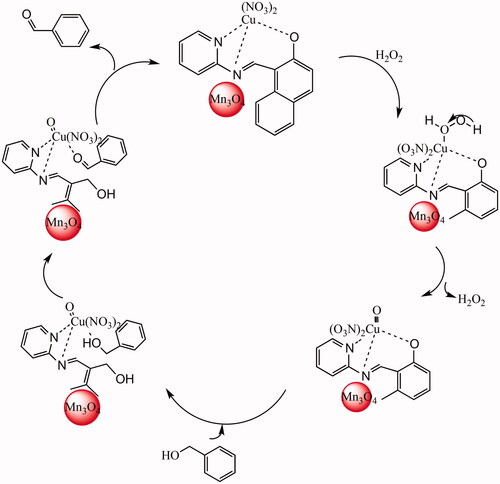
Table 1. Oxidation of alcohols catalysed by Mn3O4/CuL bio-nanocolloid using hydrogen peroxide.
Table 2. Comparison of the result of this study with that of related references.
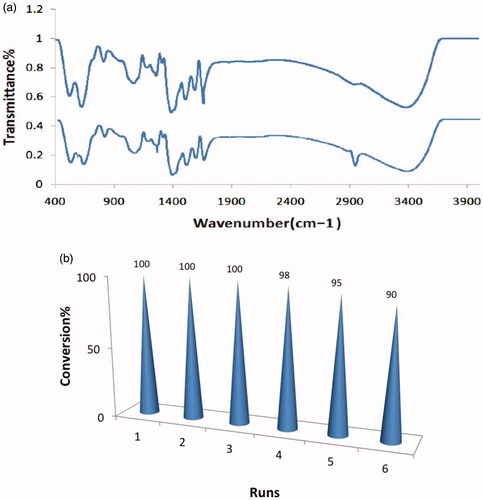
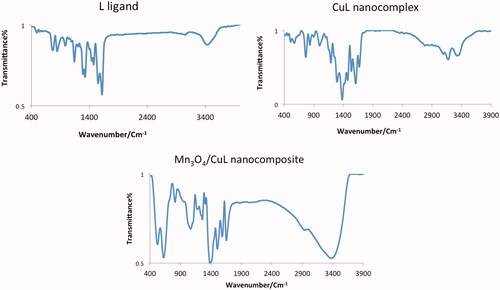
Figure 7. (a) The FT-IR of fresh catalyst (top) and reused catalyst after six times (down) and (b) recycling of catalyst.