Figures & data
Figure 1. H2O2 caused PC12 cells damage. (A) PC12 cells were received the diverse dosages of H2O2 (0–400 µM) management, CCK-8 experiment was conducted to detect cell viability. The dosage of 200 µM H2O2 was selected and utilized to manage PC12 cells. CCK-8, flow cytometry and western blot experiments were implemented to examine (B) cell viability, (C) apoptosis and (D,E) Bax, Pro/Cleaved-Caspase-3 and Pro/Cleaved-Caspase-9 expression. *p < .05, **p < .01, ***p < .001.
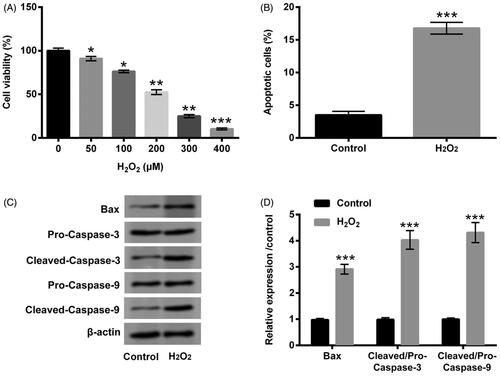
Figure 2. PA assuaged H2O2-triggered PC12 cell damage. (A) PC12 cells received the different dosages of PA (0–2 µM) administration, CCK-8 experiment was executed to test cell viability. (B) H2O2 (200 µM) and PA (0.1, 0.5 and 1 µM) were exploited to co-manage PC12 cells, CCK-8 experiment was carried out again to examine cell viability. H2O2 (200 µM) and PA (1 µM) were utilized to dispose PC12 cells, flow cytometry and western blot were conducted to evaluate (C) apoptosis and (D,E) Bax, Pro/Cleaved-Caspase-3 and Pro/Cleaved-Caspase-9 expression. *p < .05, **p < .01, ***p < .001.
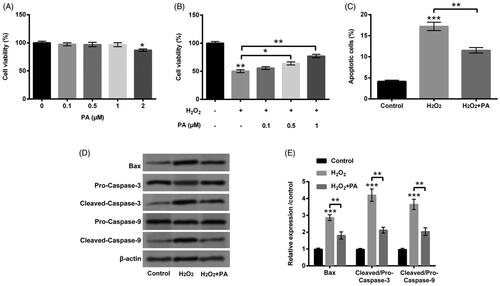
Figure 3. PA relieved H2O2-triggered PC12 cell autophagy. H2O2 (200 µM) and PA (1 µM) were utilized to dispose PC12 cells, western blot was implemented to test (A,B) protein levels of cell autophagy-associated factors (Beclin-1, p62 and LC3-II/LC3-I) in PC12 cells. **p < .01, ***p < .001.
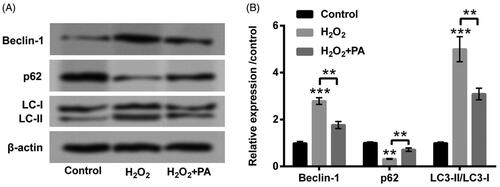
Figure 4. PA caused MEG3 down-regulation in H2O2-damaged PC12 cells. (A) PA (1 µM) was utilized to manage PC12 cells, RT-qPCR was performed to detect MEG3 expression in these disposed PC12 cells. (B) H2O2 (200 µM) and PA (1 µM) were exploited to manage PC12 cells, RT-qPCR was conducted to determine MEG3 expression in these disposed PC12 cells. (C) Expression vectors of pc-MEG3 and pcDNA3.1 were applied for PC12 cells transient transfection, and then RT-qPCR was performed again to determine MEG3 expression in these transfected PC12 cells. *p < .05, **p < .01.
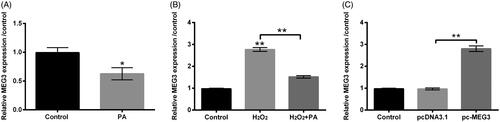
Figure 5. PA allayed H2O2-triggered PC12 cells damage through prohibiting MEG3 expression. PC12 cells received pc-MEG3 and pcDNA3.1 transfection and then were managed with H2O2 (200 µM) and PA (1 µM), CCK-8, flow cytometry and western blot were executed to test (A) cell viability, (B) apoptosis, (C,D) Bax, Pro/Cleaved-Caspase-3 and Pro/Cleaved-Caspase-9 expression and (E,F) cell autophagy-associated factors (Beclin-1, p62 and LC3-II/LC3-I) expression. *p < .05, **p < .01, ***p < .001.
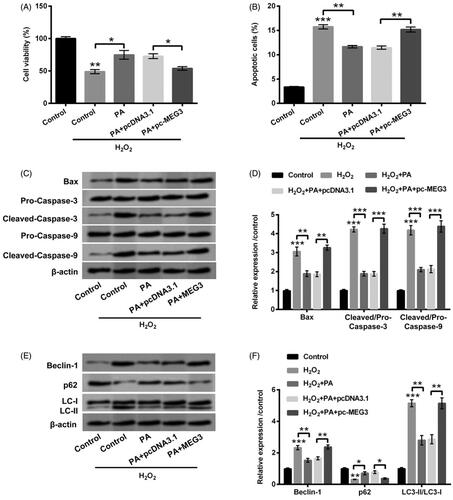
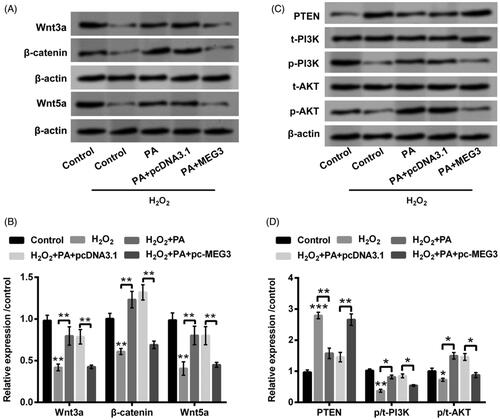
Figure 6. PA expedited Wnt/β-catenin and PTEN/PI3K/AKT activations in H2O2-managed PC12 cells through adjusting MEG3 expression. PC12 cells were received pc-MEG3 and pcDNA3.1 transfection meanwhile were managed with H2O2 (200 µM) and PA (1 µM), western blot was conducted to estimate the protein levels of (A,B) Wnt3a, β-catenin and Wnt5a as well as (C,D) PTEN, p/t-PI3K and p/t-AKT in above-managed PC12 cells. *p < .05, **p < .01, ***p < .001.