Figures & data
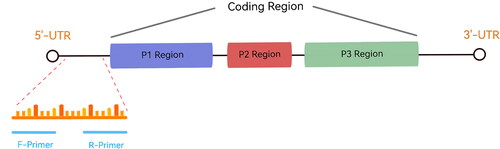
Table 1. The primers used for MIRA.
Table 2. The primers used for RT-MIRA of HAV vaccine strain H2.
Figure 2. MIRA products separated on a 2% agarose gel. L: 50-bp DNA Ladder; 1: positive control (∼250 bp); 2: primer a1 (∼180 bp); 3: primer a2 (∼269 bp); 4: primer a1, negative control; 5: primer a2, negative control.
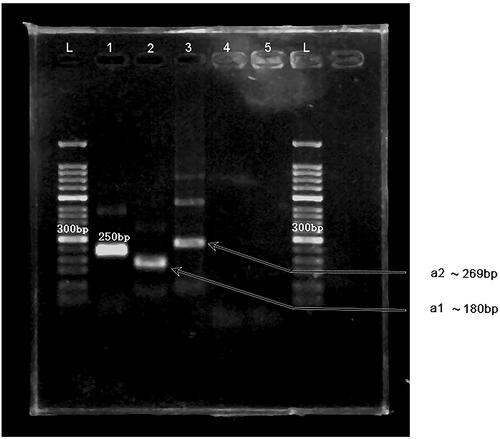
Figure 3. The MIRA products for the serial dilution of HAV plasmid DNA separated on a 2% agarose gel. L: 50-bp DNA Ladder; 1: positive control (∼250 bp); 2: 107 copies/μl template with a1 primers; 3: 106 copies/μl template with a1 primers; 4: 105 copies/μl template with a1 primers; 5: 104 copies/μl template with a1 primers; 6: 107 copies/μl template with a2 primers; 7: 106 copies/μl template with a2 primers; 8: 105 copies/μl template with a2 primers; 9: 104 copies/μl template with a2 primers.
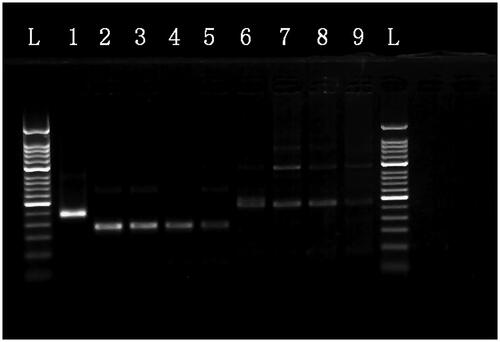
Figure 5. Cross-reactivity testing against multiple pathogens using MIRA. L: 50-bp DNA Ladder; 1: positive control (∼250 bp); 2: HAV; 3: HBV; 4: HCV; 5: HEV; 6: HIV-1; 7: HSV-1; 8: JEV; 9: rotavirus 10: human genome.
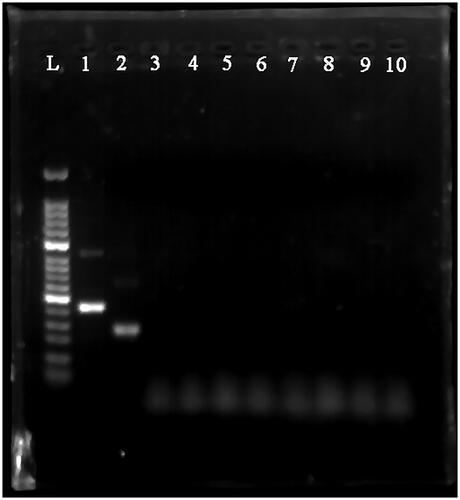
Figure 6. Serial dilution of HAV plasmid DNA detected by MIRA-LFD. 1: water template; 2: human genome template; 3: 106 copies/μl template; 4: 105 copies/μl template; 5: 104 copies/μl template; 6: 103 copies/μl template; 7: 102 copies/μl template; 8: 10 copies fg/μl template; 9: 1 copy/μl template; 10: 0.1 copy/μl template.

Figure 7. Serial dilution of HAV plasmid DNA detected by qPCR. 1: 107 copies/μl template; 2: 106 copies/μl template; 3: 105 copies/μl template; 4: 104 copies/μl template; 5: 103 copies/μl template; 6: 102 copies/μl template; 7: 10 copies/μl template; 8: water template.
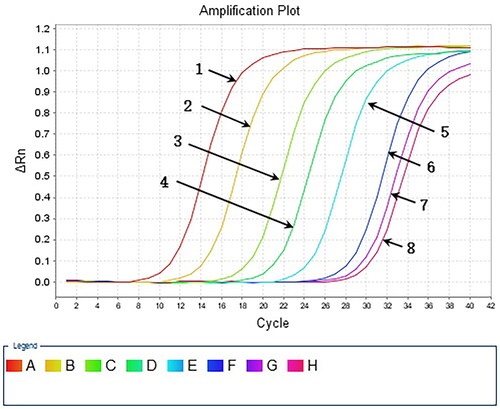
Figure 8. RT-MIRA products for the serial dilution of HAV vaccine strain H2 template separated on a 2% agarose gel. L: 50-bp DNA Ladder; 1: plasmid DNA template, positive control; 2: water template, negative control; 3: 106 copies/μl template; 4: 105 copies/μl template; 5: 104 copies/μl template; 6: 103 copies/μl template; 7: 102 copies/μl template
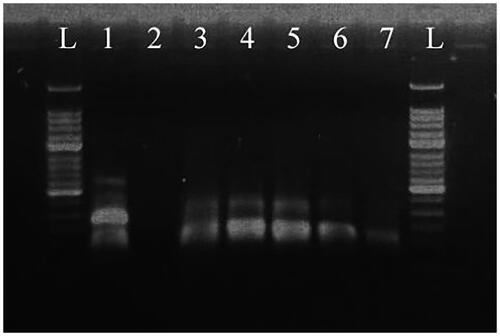
Figure 10. Serial dilution of HAV vaccine strain H2 template detected by RT-MIRA-LFD. 1: water template; 2: human genome template; 3: 105 copies/μl template; 4: 104 copies/μl template; 5: 103 copies/μl template; 6: 102 copies/μl template; 7: 10 copies/μl template; 8: 1 copy/μl template; 9: 0.1 copy/μl template.

Table 3. HAV detection in clinical samples.
Data availability statement
The data sets used and/or analysed during this study are available from the corresponding author on reasonable request.