Figures & data
Figure 1. ZIKA virus induces Hsp70 protein expression. Huh7.5 cells were infected with 3 MOI ZIKV and Hsp70 assayed by western blot at 6, 12, 24 and 48 h post-infection. Hsp70 and Hsp60 bands were quantitated using ImageJ software to calculate relative Hsp70 levels. Successful virus infection in cells was determined by detection of ZIKA E protein in the cell lysate. Hsp60 was assayed as a housekeeping control.
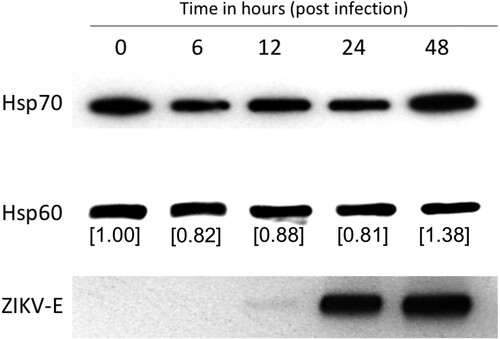
Figure 2. Hsp70 inhibitor MKT077 inhibits infectious ZIKV production. Huh7.5 cells were infected with 0.1 MOI of ZIKV. For the pre-treatment group, Huh7.5 cells were treated with 0.5, 1, and 5 µM MKT077 for 2 h and washed with DMEM before infection with ZIKV. For the post-treatment group, cells were infected with ZIKV, washed, and replenished with medium containing MKT077. Culture supernatants were collected 48 h post-infection. Virus titres in the culture supernatants were analysed by plaque assay. N = 6 per data point. Error bars denote standard error of the mean.
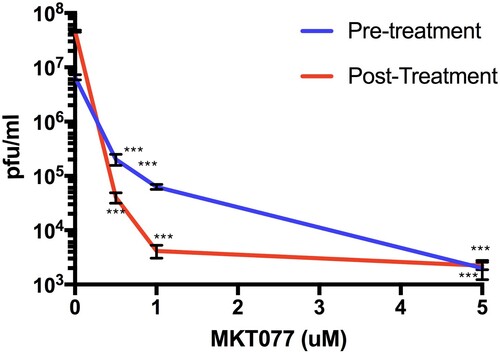
Figure 3. Heat shock 70 (Hsp70) protein facilitates ZIKV infection. Heat induction (A) in Huh7.5 cells and EGFP-Hsp70 overexpression (B) in 293 T cells was performed before infection of cells with ZIKV. Viral titres in harvested culture supernatants at 48-h post-infection were quantified by plaque assay. N = 5–6 per data point. Error bars denote standard error of the mean.
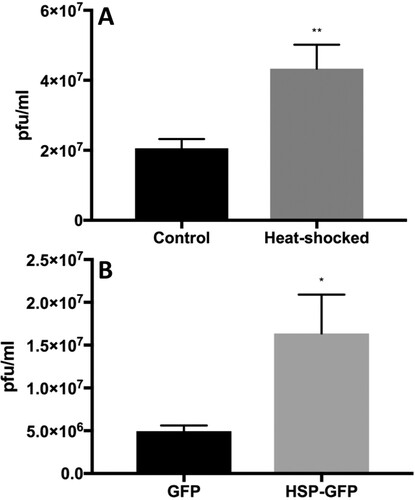
Figure 4. Intracellular and extracellular localization of Hsp70 in Huh7.5 cells. (A) The left panel shows staining of Hsp60 (cytoplasmic, mitochondrial protein) as a control for permeabilization and non-permeabilization staining conditions. The right panel shows the membrane and cytoplasmic staining of Hsp70. (B) 3-D confocal imaging of Hsp70 on the surface of unpermeablized Huh7.5 cells. Hsp70 = green. Cell nuclei = blue. Cell membrane = red.
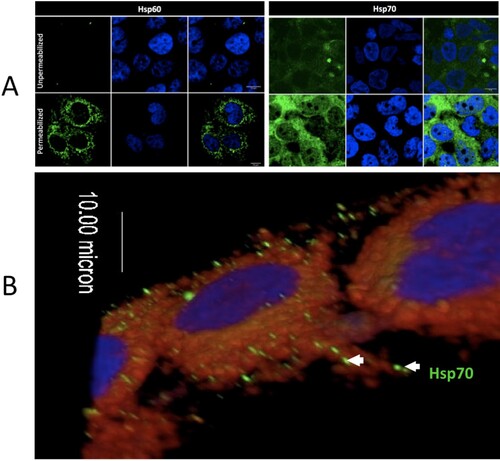
Figure 5. Blocking ZIKV cellular attachment to Hsp70 reduces cell infection rates and infectious particle production. (A) Antibody blocking of Hsp70 reduces Huh7.5 cellular infection with ZIKV. Axl antibody = positive control for blocking. Isotype antibody and beta-actin antibody = negative controls. After antibody treatment, cells were infected with ZIKV and plaque reduction neutralization assay performed. (B) Competition of ZIKV with recombinant human Hsp70 protein (rhHsp70) reduces Huh7.5 cellular infection with ZIKV. Control cells were treated with bovine serum albumin (BSA). Error bars denote 95% confidence intervals. NS = not significant.
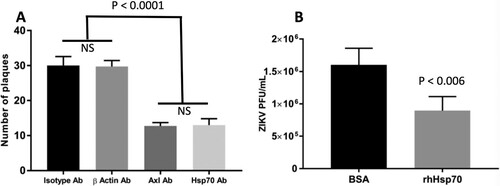
Figure 6. Interaction of Hsp70 and viral RNA. (A) Colocalization of intracellular Hsp70 and viral RNA. ZIKA virus infected Huh7.5 cells were fixed at 12-h post-infection. Cells were stained for dsRNA (green) and Hsp70 (red). Cell nuclei were stained with Hoechst 33342 (blue). Yellow dots indicate co-localized dsRNA and Hsp70. IC = ZIKV infected cell. UC = ZIKV uninfected cell. (B) Relative quantitation of ZIKA virus capsid mRNA by real-time RT-PCR in the presence of MKT077. Cells were infected with 6 MOI of ZIKV in the presence of MKT077. The Y-axis represents the expression fold-change of viral capsid mRNA normalized to vehicle (DMSO) control. Treatments with different letters are statistically different (P < .01).
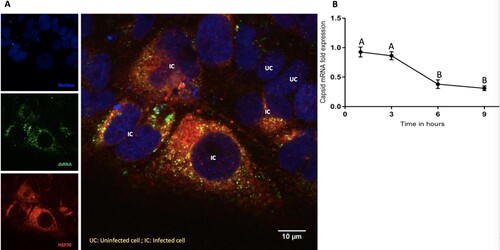
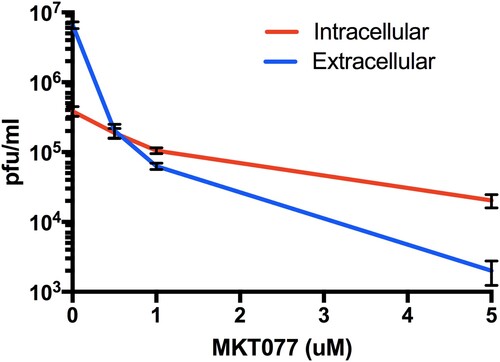
Figure 7. Role of Hsp70 in ZIKV release step. Titres of intra- and extra-cellular virus production in the presence or absence of MKT077 are shown. Following pre-treatment with 0.5, 1, and 5 µM MKT077, Huh7.5 cells were infected with ZIKV (0.1 MOI. Free virus (supernatants) and cell-associated viruses were collected 48-h post-infection. Infectious viral titres were determined by plaque assay. Error bars denote 95% confidence intervals. N = 6 per data point. Error bars denote standard error of the mean. Virus production was significantly different from between intracellular vs. extracellular compartments (P < .0001), as well as MKT007 concentration (P < .0001). There was a significant interaction term (P < .0001) noted by the crossing of the lines at 0.5 µM MKT007.