Figures & data
Figure 1. Differential NA activities from H1N1pdm influenza virus and NA-VLP. (A) NA activity of H1N1pdm virus and N1-VLP was detected and normalized using NA-Star kit. The NA activities were measured in Relative Luminescence Units (RLU) and plotted against dilutions. (B) The levels of NA protein (75 and 55 kDa) in the normalized H1N1pdm virus and N1-VLP samples were detected by western blotting using rabbit anti-NA antisera. (C) NA activities of the normalized samples against fetuin were determined in Enzyme-Linked-Lectin-Assay (ELLA). Desialylation of fetuin were detected using HRP-conjugated PNA lectin followed by OPD. Mean absorbance at 492 nm is plotted against sample dilutions. The experiments have been repeated twice with similar results.
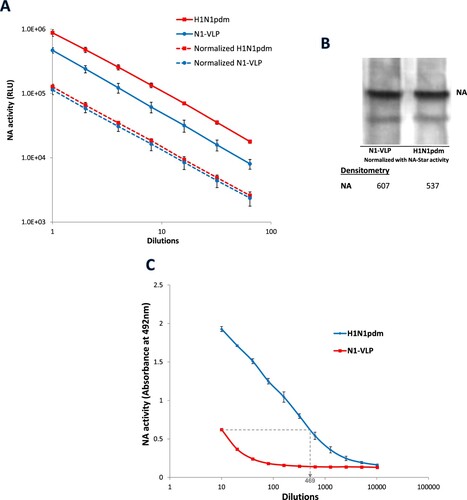
Figure 2. NA activities of NA-VLPs co-expressed with different levels of HA.H1N1-VLPs with different HA levels were collected from 293 T cells transfected with various DNA plasmids ratio and were normalized by the activities in NA-Star assays. (A) FLAG-tagged HA and NA protein levels (80 and 75 kDa) of the normalized VLPs were determined by western blotting using anti-FLAG monoclonal antibody. (B) Desialylation of fetuin by the VLPs was determined in ELLA. Mean absorbance at 492 nm is plotted against sample.
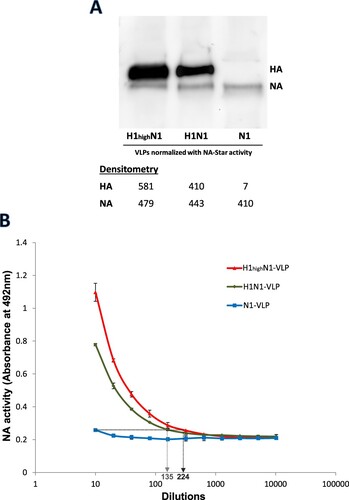
Figure 3. Effect of low pH treatment on HA and NA functions of influenza viruses.(A) Human H1N1pdm viruses and avian H9N2 influenza viruses were treated with acetate buffer at pH 6.5 or pH 5.0 and HA-receptor bindings were tested by haemagglutination of Turkey red blood cells in 2-fold dilutions. NA activities of the treated viruses (B) H1N1pdm and (C) avian H9N2 were measured by NA-Star assay or ELLA. Data from N1-VLP was included for comparison with the native H1N1pdm virus. Relative NA activities were calculated using the serial dilution curves obtained from the viruses. Mean values of 3 independent experiments were showed. *p < .05; **p < .01.
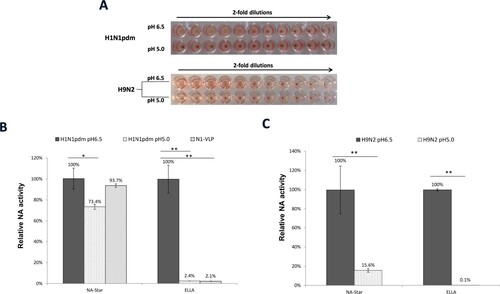
Figure 4. HA and NA functions of HANA-VLPs with deficient RBS mutation in HA. H1N1-VLPs expressing NA with either wildtype HA or ΔHA mutant (L194AY195F) from H1N1pdm were constructed and normalized with the activities in NA-Star assay. (A) HA and NA protein levels of the normalized samples were checked by western blot. (B) HA-receptor bindings were tested by haemagglutination of Turkey red blood cells in 2-fold dilutions. (C) NA activities of the VLPs against fetuin were determined in ELLA. Data obtained from N1-VLPs without HA co-expression was included for comparison. Relative NA activities were calculated using the serial dilution curves. Mean values of 3 independent experiments were showed. *p < .05; **p < .01.
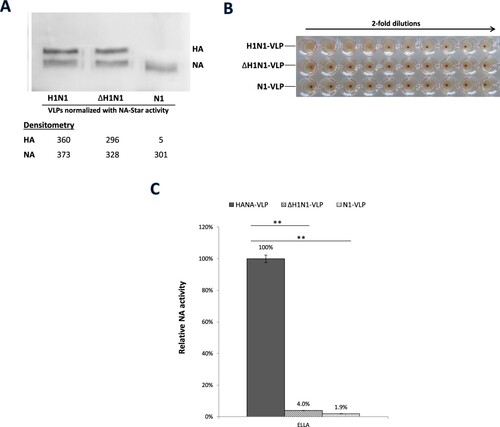
Figure 5. Desialylation of N- and O-linked glycans in fetuin by influenza virus and NA-VLP. NA activities of H1N1pdm virus and N1-VLP were measured in ELLA. (A) Desialylated N-glycans and O-glycans were detected using HRP-conjugated ECA and PNA lectins, respectively. (B) Relative NA activities at dilution 1:20 were calculated using the serial dilution curves obtained from H1N1pdm virus. Mean values of 2 independent experiments were showed. *p < .05; **p < .01.
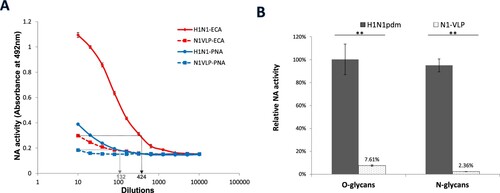
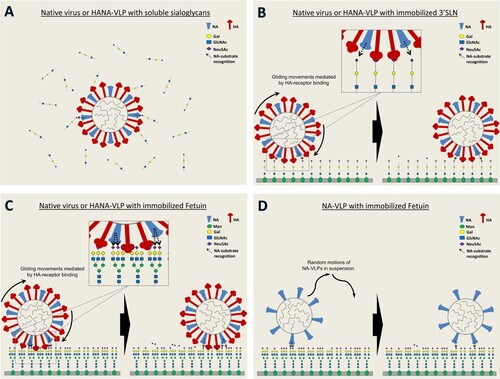
Figure 6. Desialylation of fetuin and 3′SLN by influenza virus and NA-VLP. (A) NA activities of NA-VLP and low pH pre-treated H1N1pdm viruses were measured in ELLA. Desialylation of fetuin and 3′SLN were detected using HRP-conjugated ECA lectin. (B) Cleavage of 3′SLN in solution was evaluated in NMR spectroscopy. 1H NMR spectra were recorded at 10-minute intervals. The yield of cleavage was quantified by the ratio of different chemical shift from the protons associated with NHAc group
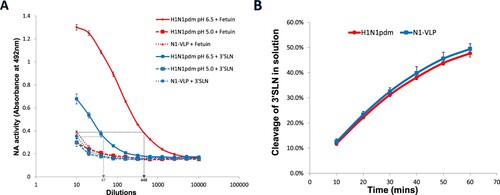
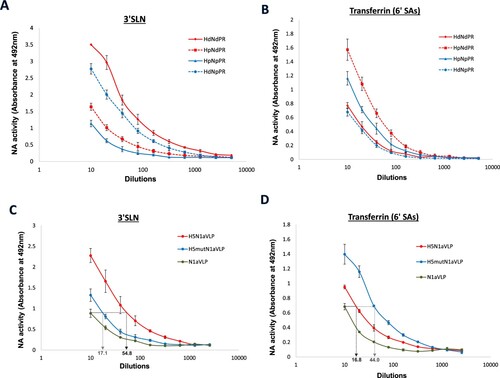
Figure 7. Desialylation of 3′SLN and human transferrin by recombinant H1N1 viruses and H5N1-VLPs. Recombinant H1N1 viruses containing HA and NA from Duck/H1N1 (Hd, Nd) or H1N1pdm (Hp, Np) were prepared by reverse genetics. NA activities of the recombinant viruses against (A) 3′SLN-BSA or (B) human transferrin were measured in ELLA. H5N1a-VLP containing HA and NA from H5N1/VN1203 were constructed along with an α2,6 SA-binding mutant (H5mut) and the NA activities against (C) 3′SLN-BSA or (D) human transferrin were measured in ELLA. Mean values of 2 independent experiments were showed.