Figures & data
Figure 1. Generation and evaluation of recombinant AdC68 expressing full-length MERS-CoV spike protein. (A) Schematic representation of the recombinant AdC68 expressing the full-length MERS-CoV spike gene (AdC68-S) (Genbank accession number: JX869059). Spike gene was inserted into the E1 region of AdC68 under the control of the CMV promoter and terminated by bovine growth hormone (BGH) polyadenylation signal sequence. (B) Western blot analysis of MERS-CoV S protein expression in 293 T cells after infection with AdC68-S (108, 109 and 1010 vp). MERS-S protein in cell lysates was probed by Rabbit anti-MERS-S1 polyclonal antibody (Sino biological). The solid arrow points to the band of S protein while the hollow arrow points to the band of S1 protein cleaved from S by the protease. (C) Cell surface expression of MERS-CoV S protein analysed by MERS-CoV-specific antibodies MERS-4, MERS-27, MERS-GD27. Cell lysates or cells infected by empty AdC68 (1010vp) were used as negative controls. 17b, an antibody against HIV-1, was used as a negative control antibody.
Figure 2. Intranasal immunization with AdC68-S induces a robust antibody and T cell response in BALB/c mice. (A) Timeline for vaccination and characterization of virologic and immunologic responses in two batches of animals. In batch I, a total of 12 groups of mice were immunized and monitored for serum binding and neutralizing activities. Group 1–6 (G1-G6) were negative controls whereas group 7–12 (G7–G12) were vaccinated with a single and varying dose of AdC68-S. In batch II, G8 and G11 mice were immunized along with G1 and G4* controls mice. These mice were examined for serum IgG subtypes, saliva IgA, and cytokine release up to 14-weeks post-immunization. The specific dose and route of immunization are indicated. i.n.: intranasal. i.m.: intramuscular. The open and solid drops indicate the blood collection for animals in the batch I and II, respectively. The temporal changes in serum binding activity to MERS-S1 (B) and neutralizing activity against autologous (C) and heterologous (D) MERS-CoV variants up to 40-weeks post-immunization are shown. Live virus neutralization by immune sera from G4, G8 (E) and G6, G11 (F) at 40-weeks post-immunization. The mean ID50 for G8 and G11 immune sera are indicated. The temporal changes in IgA in saliva (G), serum IgG subtypes (H), and cytokine release (I) in G8 and G11 animals were studied for up to 14-weeks post-immunization. Red symbols represent sera from i.n. vaccination groups and blue colour indicates sera from i.m. immunized animals. The grey colour is indicative of the control groups. ED50 means dilutions of serum at which half of the binding to antigen was identified. ID50 means dilutions of serum at which half of the viruses are neutralized. G4* indicates 1010vp used in batch I and 109 in batch II.
Figure 3 Intranasal immunization with AdC68-S provides complete protection against lethal MERS-CoV challenge in human DPP4 knock-in mice. (A) Timeline for immunization, challenge and evaluation of protective efficacy. Human DPP4 knock-in (KI) mice were immunized with either 2 × 109 vp AdC68-S or empty AdC68 via i.n. route. Ten weeks later, the same set of animals were challenged intranasally with 2000 PFU of mouse-adapted MERS-CoV strain MERS-CoV-MA and monitored daily for (B) survival and (C) weight loss. On day-4 post-infection, lung virus titres (D) were examined. Data are shown as mean ± SEM. p-values were analysed with Student’s t-test (****P < 0.0001). The dashed line in (D) indicates the limit of detection.
Figure 4. Protective efficacy of passive immunization with AdC68-S immune sera. (A) Timeline of immunization, serum transfer, challenge, and monitoring for various biological and clinical outcomes. BALB/c and human DPP4 KI mice were immunized with either 2 × 109 vp AdC68-S or empty vector AdC68 via i.n. route. 175 µl of immune sera collected from these BALB/c and hDPP4-KI mice were transferred to hDPP4-KI mice via intraperitoneal route one day before lethal 2000 PFU MERS-CoV-MA infection. (B) Neutralizing activity of immune sera from immunized BALB/c (B) and hDPP4-KI (F) mice. (C-E) Survival (C), weight loss (D) and lung viral titres (E) in hDPP4-KI mice receiving sera from immunized BALB/c mice. (G-I) Survival (G), weight loss (H) and lung viral titres (I) in hDPP4-KI mice receiving sera from immunized hDPP4-KI mice. Data are mean ± SEM. p-values were analysed with Student’s t-test (**P < 0.01; ****P < 0.0001). The dashed line in (E) (I) indicates the limit of detection.
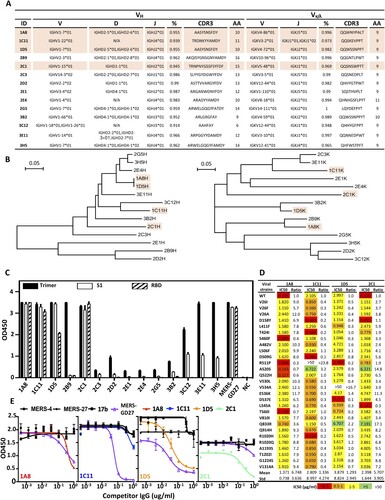
Figure 5. Characterization of AdC68-S-elicited monoclonal antibodies. (A) Summary of isolated 14 mouse mAbs on their family designations and degree of similarity compared to their germline sequences, together with their sequences of complementarity-determining region 3 (CDR3) for both VH and VL. (B) Unrooted neighbor-joining tree depicting the relationship of isolated mAbs, left panel for the heavy chain and right panel for the light chain variable region. The branch length is drawn to scale so that the relatedness between different amino acid sequences can be readily assessed. Individual sequences are named at the tip of the branches. Binding (C) and neutralizing (D) activities of 14 mAbs measured by ELISA and pseudovirus bearing naturally occurring MERS-CoV mutant strains. (E) Epitope specificity analysed by competitive ELISA. HIV-1-specific mAb 17b was used as a negative control whereas previously isolated MERS-CoV-specific human mAb MERS-4, MERS-27 and MERS-GD27 as positive controls. Data are presented as mean ± SEM. The four antibodies with potent neutralizing activities are colored in light orange in (A) and (B).