Figures & data
Table 1. Information on the two chemiluminescence immunoassays used for the diagnosis of coronavirus disease (COVID-19).
Table 2. Clinical characteristics of the 74 patients with confirmed COVID-19, stratified based on the availability of sequential samples for estimating the date of seroconversion.
Figure 1. Percentage of samples showing positive antibody findings when examined using the five studied serological tests after symptom onset.
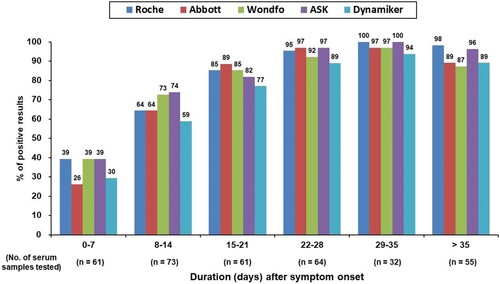
Figure 2. Chemiluminescent signal values of the two chemiluminescence immunoassays for anti-SARS-CoV-2 antibodies detection after symptom onset. (A) Roche Elecsys® Anti-SARS-CoV-2 Assay. (B) Abbott SARS-CoV-2 IgG Assay.
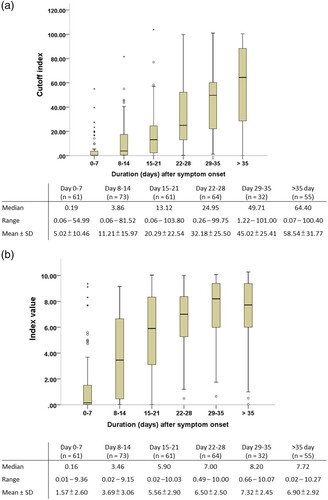
Table 3. Agreement among findings from the five anti-SARS-CoV-2 antibody tests.
Table 4. Evaluation of cross-reactivity for the five anti-SARS-CoV-2 antibody tests.
Figure 3. Parallel comparisons of the cumulative probability of seroconversion detection among the five studied serological tests.
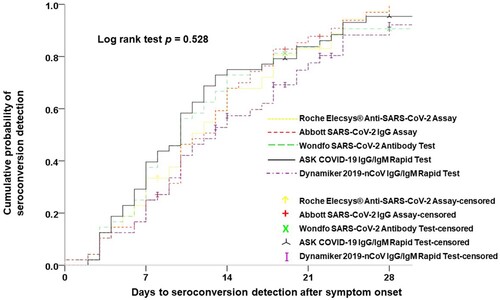
Figure 4. Detection of seroconversion in patients with COVID-19 with or without pneumonia. (A) Presumptive seroconversion based on earliest detection by any serological test. (B) Roche Elecsys® Anti-SARS-CoV-2 Assay. (C) Abbott SARS-CoV-2 IgG Assay.
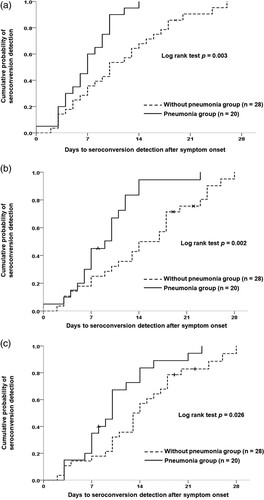
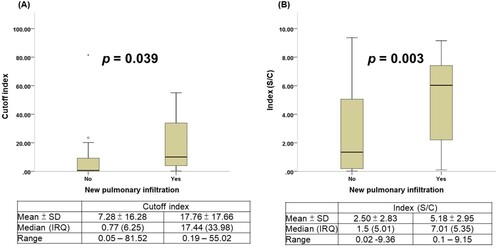
Figure 5. Comparison of chemiluminescent signal values in patients with COVID-19 with or without pneumonia. (A) Roche Elecsys® Anti-SARS-CoV-2 Assay. (B) Abbott SARS-CoV-2 IgG Assay.