Figures & data
Figure 1. Innate immune memory induced by varying stimuli. Trained immunity state can be induced by diverse agents, which enhances the immunological response of innate immune cells to either the same or unrelated secondary stimulation. Conversely, a high dosage of LPS drives innate immune cells into a tolerance state with immunological hyporesponsiveness to reinfection. Similarly, both training and tolerance states are orchestrated by epigenetic and metabolic reprogramming. Elements of some figures were made using Servier Medical Art, (https://smart.servier.com).
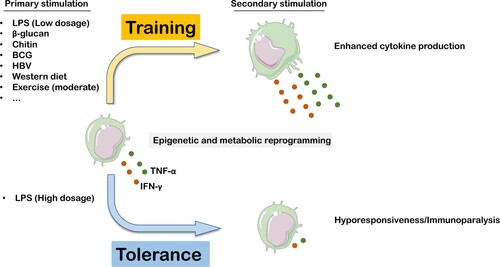
Figure 2. LPS-induced trained immunity state contributes to against Mycobacterium tuberculosis (M. tuberculosis) infection in macrophages. Following uptake by macrophages, M. tuberculosis can inhibit phagosome maturation and fusion of the phagosome with the lysosome, as well as expression levels of LC3 on the autophagosome membrane. It has been reported that M. tuberculosis can escape from the phagosome and reside in the cytosol via perforations in the phagosome membrane (data not shown). These combined efforts lead to a niche for M. tuberculosis persistence within macrophages. Activation of p62 via competitively binding of M. tuberculosis to Nrf2 with Keap1, resulting in translocation of Nrf2 into the nucleus, followed by incorporation with sMaf proteins to induce anti-oxidative cytokine expression. Interestingly, these antioxidant proteins lead to the elimination of ROS, which has an important bactericidal activity on invasive pathogens, including M. tuberculosis (left). Recent studies have found that restoration of bactericidal activities (phagocytosis, autophagy, and ROS expression) was screened in macrophages when challenged with LPS. Besides, LPS treatment can enhance the TLR4 and NOX2 expression levels. All of these effects are dependent on the TLR4-NOX2 axis (right). Elements of some figures were made using Servier Medical Art, (https://smart.servier.com).
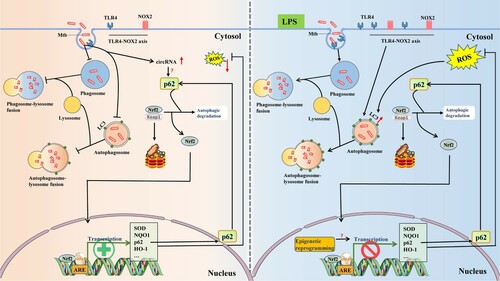
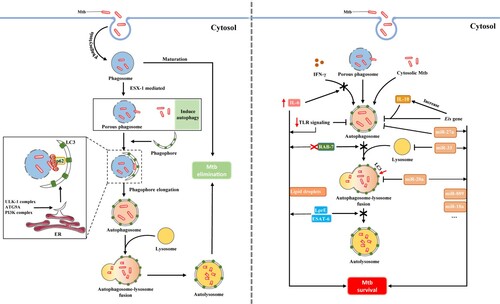
Figure 3. Mycobacterium tuberculosis (M. tuberculosis) has evolved several counterstrategies to evade autophagic elimination. Generally, M. tuberculosis can be sequestered and delivered for degradation by the phagosome upon phagocytosis by macrophages. However, M. tuberculosis can escape the phagosome and access to the cytosol via ESX-1, which subsequently activates the autophagic process, another protective degradative pathway. Cytosolic localization of M. tuberculosis with tagged ubiquitin is then delivered to phagophore formation site, ER. Further, the autophagosome fuses with the lysosome forming the autolysosome which degrades contents within. The combined effect of these events contributes to M. tuberculosis elimination (left). Unexpectedly, M. tuberculosis has evolved several countermeasures that create a niche for its survival to evade and even manipulate host autophagic processes. M. tuberculosis can directly upregulate IL-6 expression to selectively inhibit IFN-γ-induced autophagy. M. tuberculosis possesses a unique gene, Eis, by which mycobacteria arrests autophagosome formation and increase IL-10 production to block autophagy. Notably, M. tuberculosis evades autophagic elimination by using certain miRNAs (e.g. miR-20a, miR-27a, miR-33) in inhibiting autophagy. We also displayed other factors favouring mycobacterial survival in macrophages (right).