Figures & data
Table 1. Demographic and clinical features of COVID-19 patients recruited in this study.
Figure 1. Temporal profiles of IgG antibodies against S proteins of SARS-CoV-2 and HCoV-OC43.(A–B) Dynamic changes of SARS-CoV-2 S-IgG (A) and HCoV-OC43 S-IgG (B) levels in COVID-19 patient’ plasma samples over time post symptom onset measured by the plain ELISA assays (for details, see Materialans and Methods). Red and black dotted lines in violin denote the median and interquartile range of antibody titres, respectively. Non-parametric Mann–Whitney test was used for comparison of antibody titres. (C) Associations between the levels of HCoV-OC43 S-IgG and SARS-CoV-2 S-IgG in COVID-19 patient’s plasma samples. The correlation were assessed by Spearman’s rank correlation test. (D–E) Kinetics of HCoV-OC43 S-IgG levels in COVID-19 patients who had 2–5 consecutive plasma samples measured by plain ELISA assay. Each key presents the OD450 of each plasma at indicated time point.
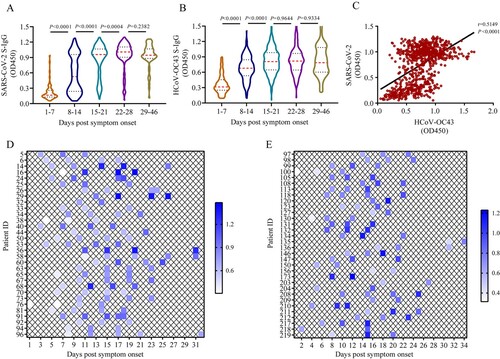
Figure 2. Two-way antigenic cross-reactivities between SARS-CoV-2 and HCoV-OC43. (A) HCoV-OC43 S-IgG levels in plasma samples taken from COVID-19 patients (n = 50) after competition by 0.5% bovine serum albumin (BSA) or SARS-CoV-2 S protein, respectively. (B) SARS-CoV-2 S-IgG levels in plasma samples taken from COVID-19 patients (n = 50) after competition using 0.5% BSA or HCoV-OC43 S protein, respectively. (C) Western blot analysis to determine the cross-reactivity between S proteins of SARS-CoV-2 and HCoV-OC43. Two human plasma samples positive for HCoV-OC43 S-IgG and two human plasma samples positive for SARS-CoV-2 S-IgG were used to probe the S proteins of SARS-CoV-2 and HCoV-OC43, respectively. Plasma samples were diluted at 1:400 and S protein of HCoV-OC43 and SARS-CoV-2 were loaded at 250 ng/well, respectively. A monoclonal antibody against SARS-CoV-2 S2 subunit (Anti-S2 mAb) was used as positive control. The red arrows indicate ectodomain and S2 subunit of S protein, respectively. Two-tailed Wilcoxon matched-pairs signed-rank test was used for antibody level comparison.
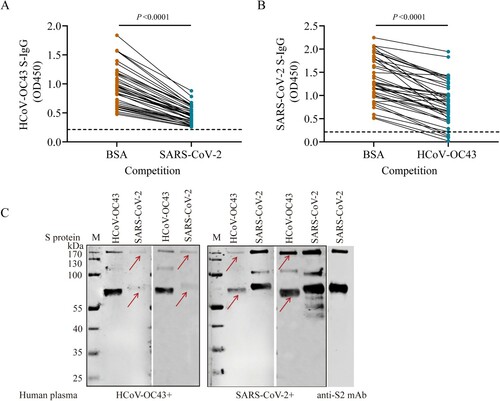
Figure 3. Cross-reactivities between SARS-CoV-2 and HCoV-OC43 S-IgG. (A–B) Cross-reactivities between HCoV-OC43 S-IgG and SARS-CoV-2 in indirect immunofluorescence assays (IFAs) (A) and Western blot assays (B). Vero cells infected with SARS-CoV-2 at a MOI of 0.1 were probed with plasma positive for HCoV-OC43 S-IgG from healthy donors (sample ID: 130, 133, 138, and 143). Plasma samples negative for HCoV-OC43 antibodies were used as negative controls (sample ID: 37 and 43). Plasma from COVID-19 patients were used as positive controls (sample ID: 192, 196). Control (in panel B): The S2 subunit were verified using a monoclonal antibody against SARS-CoV-2 S2 subunit (Control). The red arrows in panel (A) indicate Vero cells that can reacted with plasma samples positive for HCoV-OC43 or SARS-CoV-2. The red arrows in panel (B) indicate the S2 subunit of S protein. (C) Cross-neutralization between SARS-CoV-2 and HCoV-OC43 S-IgG. Neutralizing antibodies (NAbs) were measured using HCoV-OC43 S-IgG positive plasma from 50 unexposed healthy controls, and HCoV-OC43 S-IgG positive but SARS-CoV-2 S-IgG negative plasma from 50 COVID-19 patients by microneutralization assay with a SARS-CoV-2 isolate (IPBCAMS-WH-01/2019 strain). SARS-CoV-2 S-IgG positive plasma samples from 50 COVID-19 patients were used as positive control. Dashed line represents the cut-off value of NAb titres.
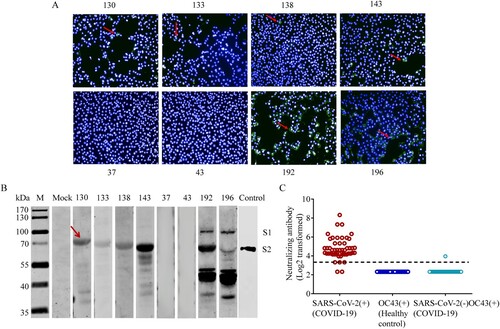
Figure 4. Cross-reactive HCoV-OC43 antibody titres correlate with disease severity in COVID-19 patients. (A) Dynamic changes of SARS-CoV-2 S-IgG levels in mild and severe COVID-19 patients. (B) HCoV-OC43 S-IgG levels in COVID-19 patients in different age groups over time post symptom onset (PSO). (C) The correlation between the HCoV-OC43 S-IgG titres and disease severity (mild and severe) in different age groups. (D) The correlation between HCoV-OC43 S-IgG titres and clinical outcome (survivor and non-survivor) in patients aged 60–85 yearsat days 1–10 PSO. (E) The correlation between HCoV-OC43 N-IgG titres and disease severity (mild and severe) in different age groups. (F) The correlation between HCoV-OC43 N-IgG titres and clinical outcome (survivor and non-survivor) in patients aged 60–85 years at days 1–10 PSO. All the antibody titres were analysed using ELISA assays and shown as mean with SD. Non-parametric Mann–Whitney test was used for comparison of antibody titres. The correlation were assessed by Spearman’s rank correlation test.
Figure 5 . Correlation between HCoV-OC43 antibody titres and disease severity in COVID-19 patients’ plasma after SARS-CoV-2 S protein competition. (A) HCoV-OC43 S-IgG antibody levels in plasma samples taken from COVID-19 patients pretreated with 0.5% bovine serum albumin (BSA) or SARS-CoV-2 S protein, respectively. Red and black dotted lines in violin denote the median and interquartile range of antibody titres, respectively. Two-tailed Wilcoxon matched-pairs signed-rank test was used for comparison of antibody titres. (B–C) HCoV-OC43-S-IgG levels in patients with mild and severe symptom (B), in patients with mechanical ventilation and non-mechanical ventilation (C) over time post symptom onset after competition using SARS-CoV-2 S protein. Non-parametric Mann-Whitney test was used for comparison of antibody titres. (D–F) The correlation between HCoV-OC43 S-IgG titres and disease severity (mild and severe) (D), mechanical ventilation and non-mechanical ventilation (E), survivor and non-survivor (F) in patients aged 60–85 years at days 1–10 post symptom onset (PSO) after competition using SARS-CoV-2 S protein. The antibody titres in (B–F) were shown as mean with SD. The correlation was assessed by Spearman’s rank correlation test.
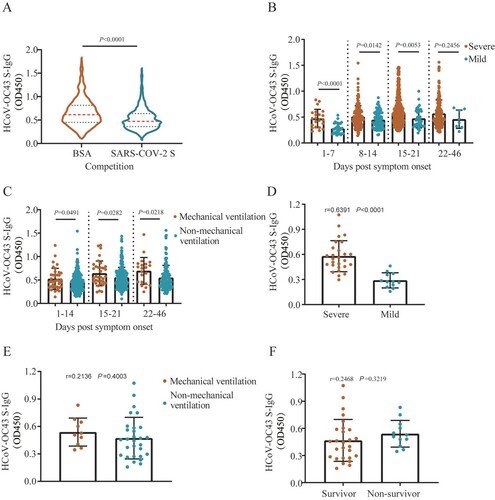
Table 2. Linear correlation between HCoV-OC43 S-IgG antibody levels and plasma concentrations of cytokines and chemokines.
