Figures & data
Figure. 1 Overexpression of SLY but not mutant SLY(P353L) enables ST1 strain (P1/7) to exhibit high hemolytic activity. (A) Expression of SLY in msly(P353L), SC-19, P1/7, P1/7-Vec, P1/7-SLY, or P1/7-mSLY was detected using western blotting with a monoclonal antibody against SLY, and unrelated protein HP0272 expression was also detected as an internal control. Densitometric analysis of expression of SLY was calculated based on the Western blot signal from the SLY/HP0272. (B) Detection of hemolytic activity of SC-19-msly(P353L), SC-19, P1/7, P1/7-Vec, P1/7-SLY, and P1/7-mSLY. The supernatant of S. suis was collected, and 1% chicken erythrocyte suspension was incubated with the supernatants for 1 h at 37°C.
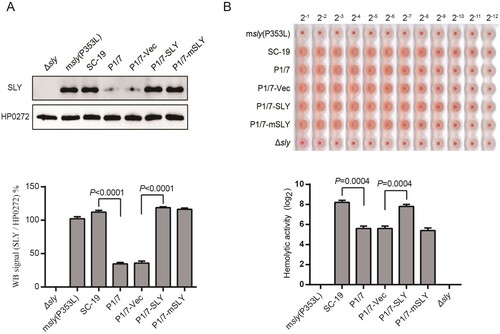
Figure 2. Overexpression of SLY but not mSLY in P1/7 causes hyperactivation of inflammasome. THP-1 cells were primed with LPS and then infected with S. suis strains SC-19, msly (P353L), P1/7-Vec, P1/7-SLY, P1/7-mSLY or stimulated with ouabain. (A) The cellular proteins and the supernatants of the cell cultures were collected for the detection of Casp1, IL-1β, and GSDMD by western blot assay. (B) The concentrations of LDH, IL-1β, and TNF-α in the supernatants of THP-1 cells were detected (n = 3).
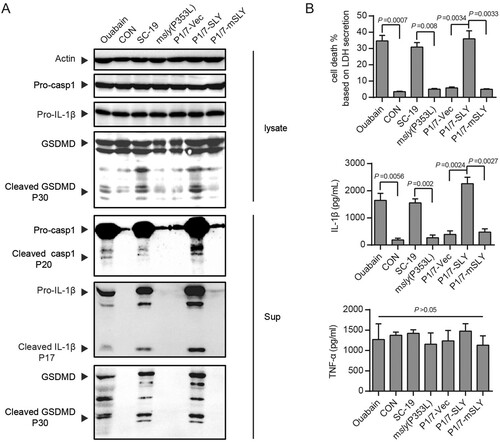
Figure 3. NLRP3 was critical to inflammasome activation in response to S. suis P1/7-SLY infection. The THP-1 nlrp3 knockout cell line (THP-1-nlrp3−/−) and its control cell line (THP-1-nlrp3+/+) were primed with LPS and then infected with S. suis strains SC-19, P1/7, P1/7-Vec, P1/7-SLY, P1/7-mSLY or stimulated with ouabain. (A) The cellular proteins and the supernatants of cell cultures were collected for the detection of NLRP3, Casp1, IL-1β, and GSDMD via western blot assay. (B) The LDH, IL-1β, and TNF-α concentrations in the supernatants were also determined (n = 3).
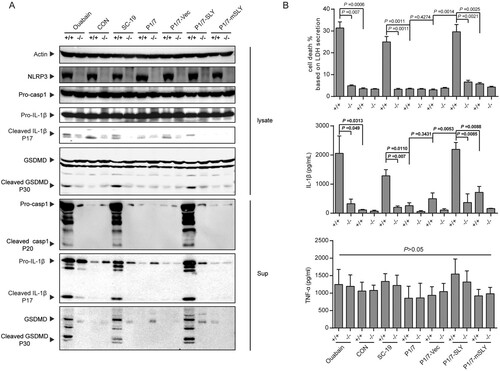
Figure 4. Evaluation of the role of overexpression of SLY in the non-epidemic S. suis strain P1/7 on STSLS with a murine model. (A) Cytokine levels in the blood at 6 h post-infection were determined (n = 4). (B) The bacterial load in the blood at 6 h post-infection was determined (n = 4). (C) Blood levels of AST, ALT, LDH, and CK at 6 h post-infection were determined (n = 4). (D) H&E staining of infected tissue sections from mice at 6 h post-infection with S. suis strains. Congestion in the lung and spleen is indicated by a “red arrow”, infiltration of inflammatory cells in the lung is indicated by a “blue arrow”, vacuolated degeneration in the liver is indicated by a “black arrow”, and necrosis in the liver is indicated by a “yellow arrow”. (E) Clinical symptom scores of mice infected with S. suis strains (n = 8). (F) Survival of mice infected with S. suis strains (n = 8). Error bars represented the mean ± standard deviations.
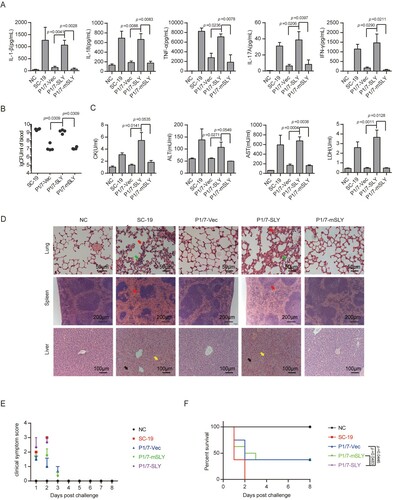
Figure 5. nlrp3 was required for STSLS caused by S. suis P1/7 overexpressing SLY. (A) Cytokine levels in the blood at 6 h post-infection were determined (n = 5). (B) The bacterial load in the blood at 6 h post-infection was determined (n = 5). (C) Blood levels of AST, ALT, LDH, and CK at 6 h post-infection was determined (n = 5). (D) H&E staining of infected tissue sections from mice at 6 h post-infection with S. suis strains. Congestion in the lung and spleen is indicated by a “red arrow”, infiltration of inflammatory cells in the lung is indicated by a “blue arrow”, vacuolated degeneration in the liver is indicated by a “black arrow”, and necrosis in the liver is indicated by a “yellow arrow”. (E) Clinical symptom scores of mice infected with S. suis strains (n = 10). (F) Survival of mice infected with S. suis strains (n = 10). Error bars represented the mean ± standard deviations.
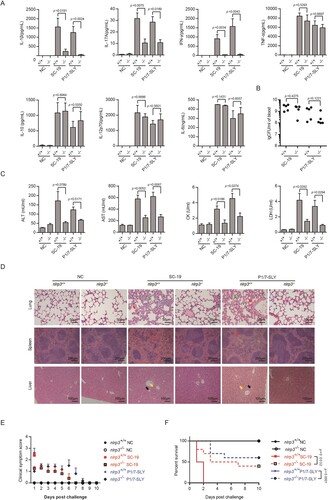
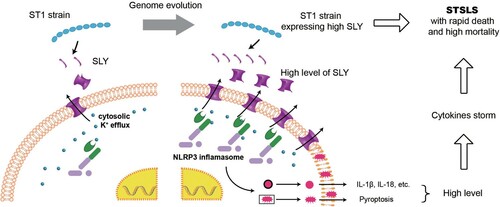
Figure 6. Acquiring high expression of SLY enabled non-epidemic S. suis to cause STSLS through NLRP3 inflammasome hyperactivation. It also provided an explanation why the epidemic S. suis strain, which evolved from ST1 strain and expressed high level of SLY, could suddenly cause a cytokines storm and STSLS.