Figures & data
Figure 1. Characterization of genotype I ASFV isolates in vitro. PAMs were infected with the genotype I isolates SD/DY-I/21 and HeN/ZZ-P1/21, and the genotype II strain HLJ/18 as a control at an MOI of 0.1. The cells were fixed and analysed by using an immunofluorescence assay (IFA) at 24 h p.i. The haemadsorption (HAD) assay was performed with the indicated viruses in PBMCs. PAMs were infected with ASFVs in 6-well plates (MOI=0.2), and the cell pellets were harvested for morphological assessment by using an electron microscope (EM).
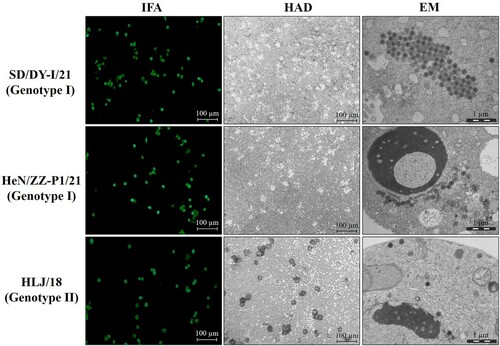
Figure 2. Phylogenetic tree based on the full genome sequences derived from SD/DY-I/21, HeN/ZZ-P1/21, and 70 reference strains from the GenBank database (accession numbers are reported in brackets). GI, all strains are genotype I ASFVs. GII, all strains are genotype II ASFVs. The red boldface type indicates the isolates in this study.
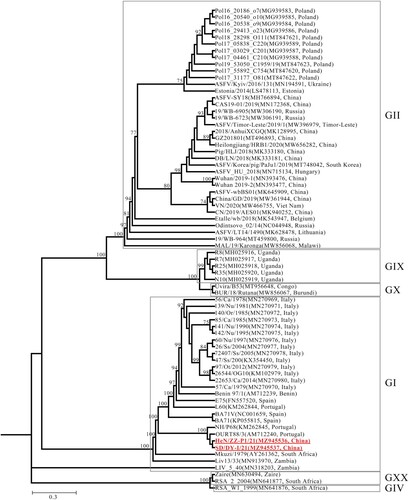
Figure 3. ORFs, nucleotide mutations, deletions, insertions, and replacement in the genomes of SD/DY-I/21 and HeN/ZZ-P1/21. Analysis of the deletion, shortening and lengthening of all ORFs of SD/DY-I/21 and HeN/ZZ-P1/21 compared with virulent isolates L60 and Benin 97, and attenuated isolates NH/P68 and OURT88/3 (A). The whole genome sequences of SD/DY-I/21 and HeN/ZZ-P1/21 were respectively compared with those of low virulence isolates NH/P68 and OURT88/3 for nucleotide mutations in ORFs (B), nucleotide deletions, insertions, and replacement in ORFs (C); and nucleotide mutations, deletions, and insertions in the noncoding regions (D). The names of the ORFs are shown on the bottom of each panel.
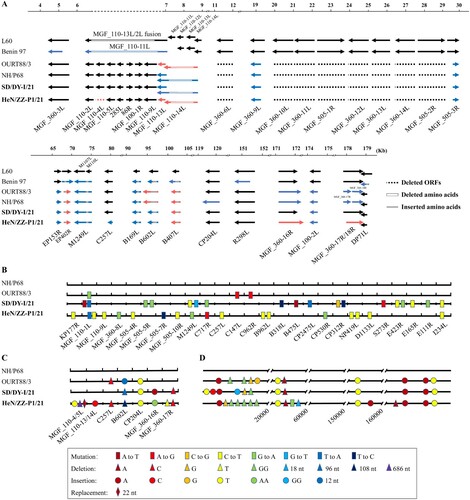
Figure 4. Rectal temperature changes in pigs infected with SD/DY-I/21 at a dose of 106 TCID50 (A) or 103 TCID50 (B). Pig 1–Pig 6, 6 pigs inoculated with SD/DY-I/21, Contact 1–Contact 4, 4 non-inoculated pigs cohoused to test for contact transmission. The dashed black lines in these panels indicate the threshold of normal rectal temperature.
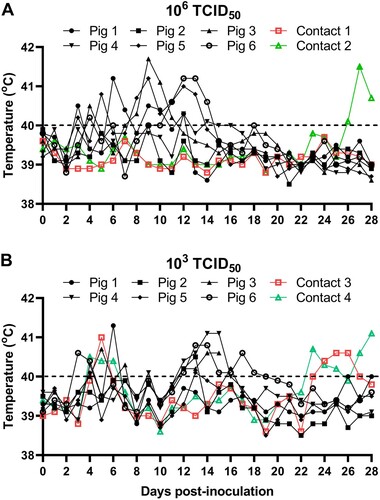
Figure 5. Disease signs in pigs infected with the genotype I isolate SD/DY-I/21. Disease signs include papules (A,B), cutaneous necrosis (B,C), and arthroncus of hind legs (B,D) in surviving pigs.
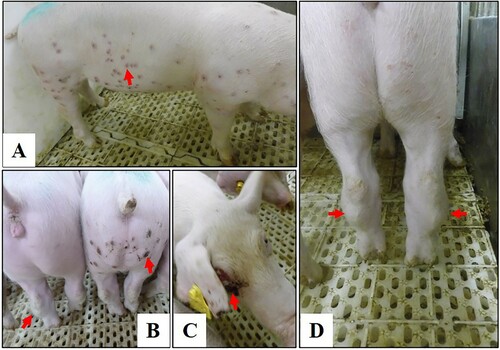
Table 1. Disease signs in pigs inoculated with different doses of the genotype I ASFV SD/DY-I/21.
Figure 6. Detection of virus shedding and viremia in infected and contact pigs by use of qPCR. Oral and rectal swab samples, as well as blood, were collected from pigs infected with SD/DY-I/21 and contact pigs at the indicated days post-infection. Viral DNA was extracted and detected by using qPCR. The data on the contact pigs cohoused with the 106 TCID50-inoculated pigs and 103 TCID50-inoculated pigs are labelled in red and blue, respectively. The different shaped black dots represent individual pigs.
Figure 7. Detection of virus load in tissues and serum conversion in SD/DY-I/21-infected pigs and contact pigs. (A) The indicated tissue samples were collected from the dead pig and surviving pigs that were euthanized on Day 28 post-inoculation or post-contact to detect viral DNA by using qPCR. LN1, intestinal lymph node; LN2, inguinal lymph node; LN3, submaxillary lymph node; LN4, bronchial lymph node; LN5, gastrohepatic lymph node; and LN6, mediastinal lymph node. (B) ASFV-specific antibody in sera from infected and contacted pigs was detected at the indicated times post-infection or -contact by using a commercial ELISA kit coated with viral p72 protein. The data on the contact pigs cohoused with the 106 TCID50- and 103 TCID50-inoculated pigs are labelled in red and blue, respectively. The different shaped black dots represent individual pigs.