Figures & data
Table 1. Basic characteristics of participants.
Figure 2. Immune response after the third-dose vaccination. (a) IFN-γ SFU/million PBMCs after the third-dose vaccination. (b) Humoral immune responses against prototype and variants of SARS-CoV-2 after the third-dose vaccination evaluated by pVNT. (c) Humoral immune responses after the third-dose vaccination evaluated by sVNT. (d) Humoral immune responses after the third-dose vaccination evaluated by an anti-RBD antibody. (e) Humoral immune responses after the third-dose vaccination evaluated by anti-RBD IgG.
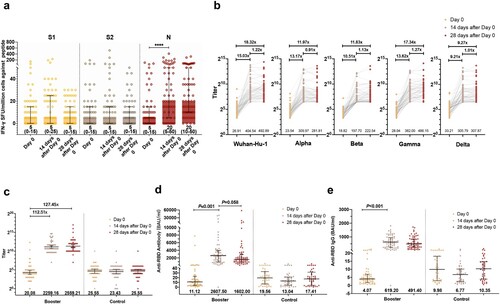
Figure 3. Impact of the interval among three doses of BBIBP-CorV vaccinations on humoral immune responses and T-cell response. (a) pVNT titer against prototype and variants of SARS-CoV-2 in participants administered with third doses at 4–6 months and 7–8 months intervals after second doses; (b) T-cell response in participants administered with third doses at 4–6 months and 7–8 months intervals after second doses.
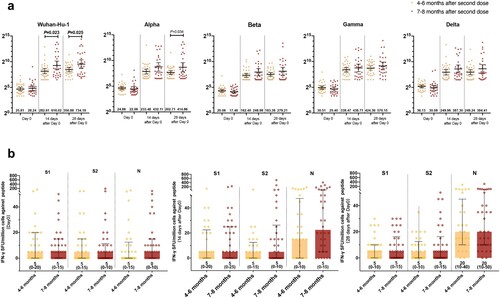
Table 2. Solicited and unsolicited adverse reactions.