Figures & data
Table 1. Susceptibility of Aedes aegypti mosquitoes from Mombasa, Kisumu, and Nairobi to oral infection with yellow fever virus.
Table 2. Susceptibility of Aedes bromeliae mosquitoes from Mombasa and Nairobi to oral infection with yellow fever virus (YFV).
Table 3. Susceptibility level of Aedes mosquitoes inoculated intrathoracically with yellow fever virus.
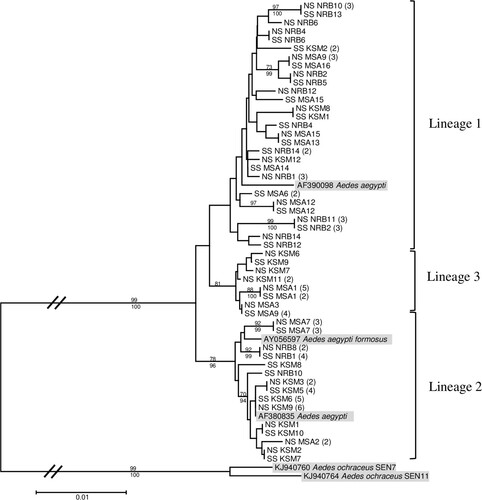
Figure 1. Maximum likelihood tree inferred using the (T92 + G + I) model of sequence evolution for COI barcode region (860 bp) of yellow fever midgut infected (SS) and non-infected (NS) Ae. aegypti samples from Nairobi (NRB), Kisumu (KSM), and Mombasa (MSA), Kenya. The number of individuals sharing a haplotype is indicated in parentheses. Bootstrap support values from 1000 replications ≥65 and Bayesian posterior probabilities ≥90 are indicated above and below the three major lineages, respectively, with terminal nodes reflecting bootstrap support values alone. Aedes ochraceus was included as an outgroup.