Figures & data
Figure 1. The N, S and RBD protein-specific IgG antibody response in SARS-CoV experienced and SARS-CoV naïve vaccinees. Serum samples were collected at 28 days after the first dosage (D28) and 28 days after the second dosage (D56). (A-C) serum IgG binding antibodies against SARS-CoV-2 S, RBD, and N proteins were measured by ELISA; (D-F) serum IgG binding antibodies against SARS-CoV S, RBD, and N proteins were measured by ELISA. The half-maximal titre (EC50) was calculated using four-parameter logistic curve fit. Red squares and lines represent SARS-CoV experienced vaccinees. Blue circles and lines represent SARS-CoV naïve vaccinees. The pink and blue lines represent the median values of the SARS-CoV experienced vaccinees and the SARS-CoV naïve vaccinees, respectively. The dashed black lines represent the positive cutoff value. Statistical significance was determined using one-way ANOVA-test. * denotes p < 0.05, ** denotes p < 0.01, *** denotes p < 0.001, **** denotes p < 0.0001, ns, non-significant.
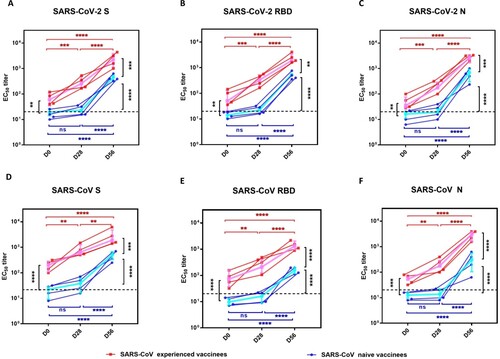
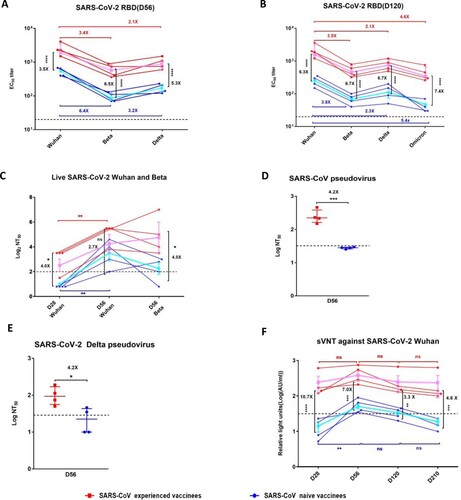
Figure 2. Neutralizing antibody responses against SARS-CoV-2 and SARS-CoV in SARS-CoV experienced and SARS-CoV naïve vaccinees. After the first dosage, serum samples were collected at day 28, day 56, day120, and day210. A second dosage was given at day 28 after the first dosage. (A) Serum IgG binding antibodies against RBDs of Wuhan, Beta, and Delta on day 56 after the first dosage. (B) Serum IgG binding antibodies against RBDs of Wuhan, Beta, Delta, and Omicron on day 120 after the first dosage. (C) Serum neutralizing antibody titres to live SARS-CoV-2. Wuhan and Beta on day 28 after the first dosage(D28) and day 28 after the second dosage(D56). (D) Serum neutralizing antibody titres to pseudotype SARS-CoV on day 28 after the second dosage(D56). The half-maximal neutralizing titre (NT50) was measured by a pseudovirus-based neutralizing assay and calculated by Reed Muench. (E) Serum neutralizing antibody titres to VSVpseudotype SARS-CoV-2 Delta on day 28 after the second dosage(D56). (F) A CLIA-based surrogate virus neutralization test (sVNT) to measure the RBD-targeted neutralizing antibodies that compete with the binding hACE2. Red squares and lines represent SARS-CoV experienced vaccinees. Blue circles and lines represent SARS-CoV naïve vaccinees. The pink and blue lines represent the median values of the SARS-CoV experienced vaccinees and the SARS-CoV naïve vaccinees, respectively. The dashed black lines represent the positive cutoff value. Statistical significance was determined using one-way ANOVA-test. * denotes p < 0.05, ** denotes p < 0.01, ***denotes p < 0.001, **** denotes p < 0.0001, ns = non-significant.