Figures & data
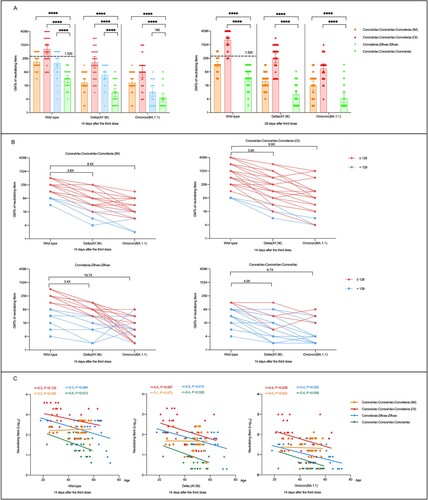
Figure 1. Neutralizing antibodies to wild-type SARS-CoV-2, Delta, and Omicron (BA.1.1) variants after receiving heterologous or homologous prime-boost immmunization regimens. (A) Horizontal dotted line, the WHO reference NIBSC code: 20/136 (1000 IU ml−1 in serum) is equivalent to a live viral neutralizing antibody titer of 1:320 against wild-type SARS-CoV-2. Statistical comparisons were performed by using GMTs of heterologous booster groups (CoronaVac-CoronaVac-Convidecia (intramuscular injection, IM), CoronaVac-CoronaVac-Convidecia (orally inhaled, OI), Convidecia-Zifivax-Zifivax) versus that of homologous booster group (CoronaVac-CoronaVac-CoronaVac), respectively; ****P< 0.0001; NS, not statistically. (B) The values are the folds reduction of GMTs of neutralizing antibodies against SARS-CoV-2 wild-type and Delta versus Omicron variants 14 days after the third dose. Red dots represent participants with a neutralizing titer≥1:128 against wild-type SARS-CoV-2. Blue dots represent participants with a neutralizing titer < 1:128 against wild-type SARS-CoV-2. C, Correlation of age and neutralizing antibodies titers to wild-type SARS-CoV-2, Delta, and Omicron variants 14 days after the last boosting dose by four immunization regimens.