Figures & data
Figure 1. Immune responses to ASFV infection. Both humoral and cellular immune responses appear to be important for protection against ASFV infection. T cells have been shown to play a particularly important role in survival with key roles identified for natural killer (NK) and CD8+ T cells. CD4+ T helper cells seem to support B cell responses and essential antibody (Ab) maturation, particularly in infection with highly virulent isolates. Studies on nonconventional T cells, such as effector γδ T cells and invariant Natural Killer T (iNKT) cells, indicate these cell subsets also take part in the antiviral response against ASFV. In wild boar, the significant bias towards γδ T cells has been suggested as an explanation for the higher disease severity and lethality in this species. Several studies have also revealed the relevance of antibodies in the protection against ASF. Antibody-mediated neutralization has some uncommon characteristics in ASFV infection, namely loss of susceptibility to neutralization by cell culture passage because of changes in the phospholipid composition of viral membranes and/or the presence of sera blocking antibodies that inhibit complete neutralization. A number of ASFV proteins have been implicated in the induction of neutralizing antibodies during infection, most notably the ASFV hemagglutinin CD2v/EP402R. Other antibody driven protective mechanisms include antibody-dependent cell-mediated cytotoxicity (ADCC) and complement-mediated cytotoxicity (CDC). Created with BioRender.com (accessed on 01 July 2022).
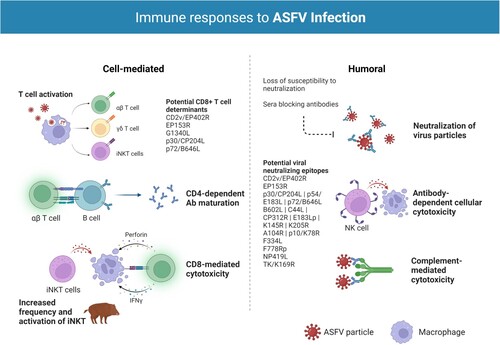
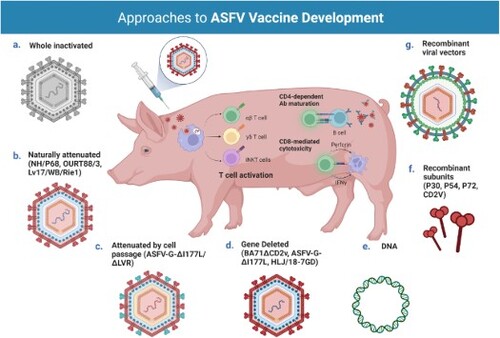
Figure 2. Different approaches for the development of vaccine candidates against ASFV. Three main strategies have been employed in the development of ASFV vaccine candidates: whole inactivated ASF virus vaccines; live virus-vectored recombinant, subunit, and mammalian expression plasmid vaccines; and live attenuated virus vaccines (LAVs), which we have further subcategorized into naturally attenuated or attenuated by cell passage, and gene deleted vaccines. The figure highlights the main advantages and disadvantages of each approach, as well as existing examples under development. DIVA – Differentiating Infected from Vaccinated Animals. Created with BioRender.com (accessed on 01 July 2022).
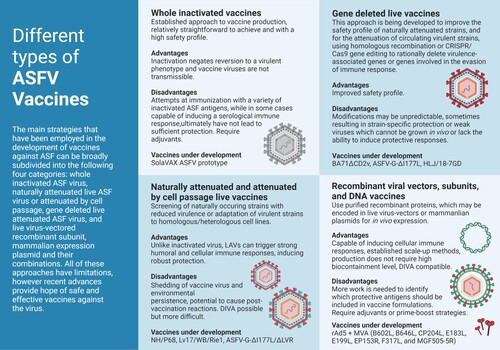
Table 1. Summary of the number of outbreaks and animal losses caused by ASF in the different world regions (2016-2022). Data reported since January 2020 covers only epizootic situation. * Losses (deaths + animals killed and disposed of): this figure refers to losses in the establishments affected by the outbreaks and it does not include the animals culled in areas around the outbreak for controlling the disease. Source WHOA/OIE WAHIS.
Table 2. Promising Live Attenuated Vaccines developed in 2015–2022. PBMs porcine blood monocyte/macrophages; BMs pig bone marrow cells; COS-1 monkey kidney tissue-derived cells; PAMs primary porcine alveolar macrophages; PIPEC Plum Island porcine epithelial cells, a porcine fetal kidney cell line engineered to express the bovine αVβ6 integrin.