Figures & data
Table 1. Primers used for generating mutant furin cleavage site SADS-CoV S.
Figure 1. The swine acute diarrhea syndrome coronavirus (SADS-CoV) spike (S) protein induces cell – cell fusion in various cell types. (A) Schematic representation of the cell-cell fusion assay (see details in Materials and Methods). (B, C) Cells were transfected with SADS-CoV S-C9 or empty vector (control) and green fluorescent protein (GFP). After 48 h, cell – cell fusion was observed by fluorescence microscopy (B) and scored as 0, 1, 2, or 3, representing 0%, less than 33%, 33% to 66%, and more than 66% fuzed cells (C). Scale bars, 100 µm. (D) S expression was examined by western blot analysis using an anti-C9 antibody.
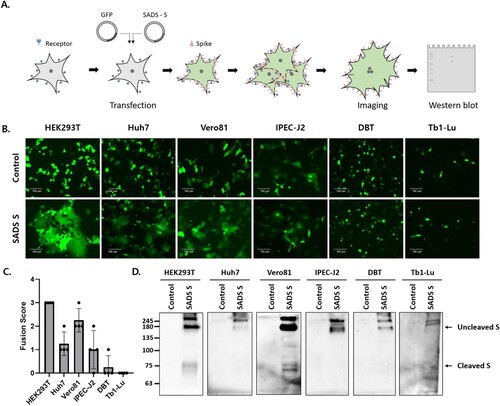
Figure 2. Furin activates SADS-CoV S-mediated cell – cell fusion and S cleavage. (A, B) HEK293T cells were transfected with SADS-CoV S and GFP. After 6 h, transfected cells were treated with the indicated protease inhibitors. Cell – cell fusion was observed by fluorescence microscopy (A). Scale bars, 100 µm. S expression was examined by western blotting (B). (C) Cells were transfected with SADS-CoV S and GFP in the presence or absence of human furin. After 48 h, cell nuclei were stained with DAPI and cell – cell fusion was assessed by fluorescence microscopy. Scale bars, 100 µm. (D) Nuclei in the fuzed cells were counted. Statistical significance was assessed by Student’s t test. ***, P < 0.001.
Figure 3. S cleavage at two furin cleavage sites is required for cell – cell fusion. (A) Schematic showing the SADS-CoV S protein containing a receptor binding domain (S1) and a fusion domain (S2). The amino acid (aa) residues of three putative furin cleavage sites and the S2` cleavage sites are indicated, and the mutations introduced into the cleavage sites are shown in bold. (B) HEK293T cells expressing wild type (WT) and mutant S proteins along with GFP. Cell – cell fusion was observed by fluorescence microscopy. Where indicated, cells were treated with 5 µg/mL trypsin. Scale bars, 100 µm. (C) S expression was examined by western blotting.
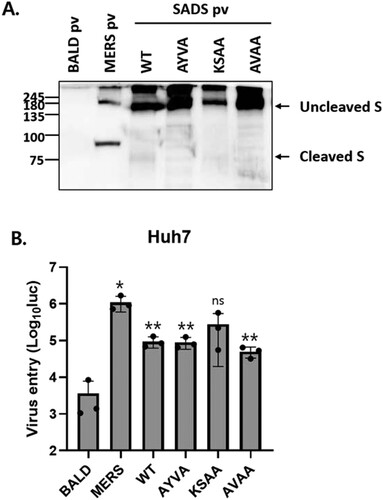
Figure 4. SADS-CoV S cleavage by furin is not required for viral entry. (A) S proteins in pseudotyped viruses (pv) were examined by western blotting. BALD pv indicates pseudotyped viruses lacking S proteins. (B) Huh7 cells were transduced with the indicated pseudotyped viruses. After 48 h, viral entry was quantified by measuring luciferase levels. Statistical significance was assessed by Student’s t test. *P < 0.05; **P < 0.01; ***P < 0.001; ns, not significant.