Figures & data
Figure 1. The progression of COVID-19 and potential damage. Left, SARS-CoV-2 infects the human airway epithelium via ACE2 receptors, which are mainly located on type II pneumocytes. In mild disease, the recruitment of myeloid cells occurs without causing an inflammatory storm, and the virus is cleared. Right, in severe COVID-19, SARS-CoV-2 escapes from the immune system, penetrates the epithelium, and infects endothelial cells that induce cell debris. Severe COVID-19 involves diffuse alveolar damage, endotheliitis, and fluid overload associated with neutrophils originated cytokine storm and NETs. The image was created with BioRender.com.
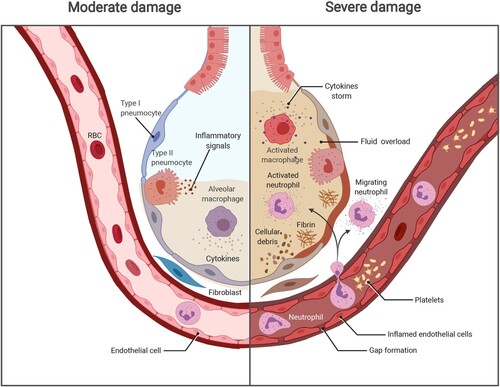
Figure 2. The hypothesis of how NETs lead to lung damage in COVID-19. First, SARS-CoV-2 infects the human airway epithelium via binding to ACE2 or TMPRSS2 receptors by invading type II pneumocytes. Insufficient PAMP/DAMP recognition is induced by viral evasion strategies and leads to inflammatory cytokine release. The inflammatory cytokines recruit neutrophils into viral-infected tissues in the lungs. The excessive inflammatory infiltration causes a cascade of cytokines (e.g. TNF-α, IL-6, IL-8), also called hyper-inflammation. Neutrophils involved in cytokine storm, on the other hand, lead to NET overproduction, represented by target proteins such as PAD4 as well as NE, priming the procedure of NETosis. Finally, the lung epithelium is injured by the processes of NETosis, reactive oxygen species, and myeloperoxidase overaction. The image was created with BioRender.com.
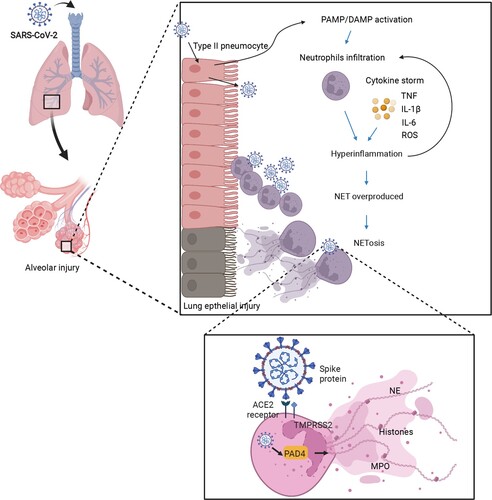
Figure 3. The hyperactivation of monocyte-derived macrophages in COVID-19. After SARS-CoV-2 infection, a delayed IFN response is induced, owing to the anti-IFN response triggered by the viral protein components. The insufficient IFN response leads to a failure of viral clearance by innate immunity, causing a recruitment of circulating blood cells, such as monocytes, and chemokine release. Moreover, those released chemokines, including CCL1 and CCL7, further provoke the monocytes into an inflammatory state, known as monocyte-derived inflammatory macrophages in the lungs with sustained PAMP/DAMP activation and NLRP3 inflammasome formation. These types of cells kill the lung epithelia either via the NLRP3 inflammasome activated-caspase pathway or oxidative stress reaction and enhance the cytokine storm by antibody-dependent enhancement. The image was created with BioRender.com.
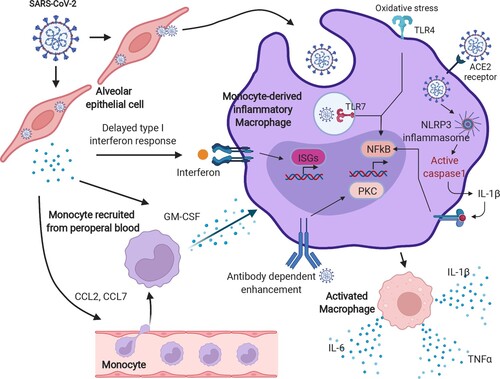
Figure 4. The imbalance in T-cell immunity between severe and mild COVID-19. The dysregulation of T-cells includes imbalanced immune cell compartments and the expression of inhibitory molecules such as PD-1, Tim-3, and NKG-2A. The dynamic disease progression from mild to severe symptoms, which manifested with cytokine storm, was exhibited with distinct T subsets and T-cell responses. In mild COVID-19 cases, the Th1 subsets were higher than those in severe COVID-19 cases, leading to a strong CTL and macrophage phagocytes. However, in severe cases, Th2, Th17, and Treg subsets were comprised mainly of T-cells, together also with highly expressed inhibitory molecules, such as PD-1, TIM-3, and NKG2A. The image was created with BioRender.com.
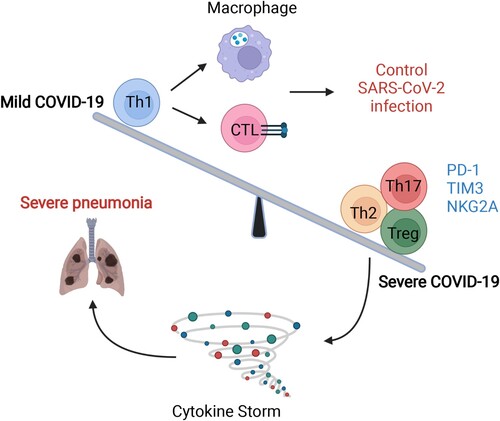
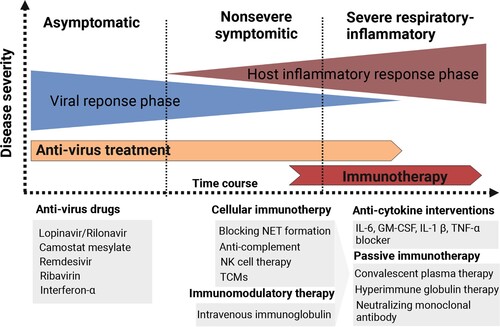
Figure 5. Different immunotherapy strategies for COVID-19 patients. Anti-virus drugs and multiple types of immunotherapeutic targets against severe COVID-19 patients are illustrated. The image was created with BioRender.com.